Evaluation of the Analytical Performances of the Biolabo SOLEA 100 Optical Coagulometer and Comparison with the Stago STA-R MAX Analyser in the Determination of PT, APTT, and Fibrinogen
- PMID: 36611377
- PMCID: PMC9818767
- DOI: 10.3390/diagnostics13010085
Evaluation of the Analytical Performances of the Biolabo SOLEA 100 Optical Coagulometer and Comparison with the Stago STA-R MAX Analyser in the Determination of PT, APTT, and Fibrinogen
Abstract
Introduction: The Biolabo Solea 100 is a fully automated coagulation analyser using an optical system to detect coagulation designed to meet the needs of small- and medium-sized laboratories. This study aimed to evaluate the analytical performance in terms of bias, precision, and interference of the Biolabo Solea 100 coagulometer under routine laboratory conditions. In addition, a comparison was made with Stago STA-R MAX.
Materials and methods: Imprecision and bias were evaluated for activated partial thromboplastin time (APTT), fibrinogen (FIB), and prothrombin time (PT) at the medical decision levels. The results of 200, 181, and 206 plasma samples for APTT, FIB, and PT, respectively, were compared with those obtained by Stago STA-R MAX. In addition, the interference level of bilirubin, haemoglobin, triglycerides, and fractionated heparin was evaluated.
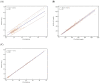
Results: Repeatability, intermediate imprecision, bias, and total error are overall below the defined limits of acceptability. Of interest is the high degree of agreement between Solea 100 and STA-R MAX with respect to PT (s), which fits perfectly with the theoretical line of identity (y = 0 + 1.00x). No interferences were found within the limits stated by the manufacturer, with some exceptions for APTT with heparin and APTT and PT for higher bilirubin concentrations.
Conclusions: In conclusion, the performance of the Solea 100 optical analyser is satisfactory and adequate for the determination of routine coagulation tests. Moreover, they are perfectly comparable to mechanical systems, such as STA-R MAX and other upper-level analysers, even considering the low interference levels under routine conditions.
Keywords: analytical techniques and equipment; haematology and coagulation; mechanical clot detection; optical clot detection; validation/evaluation.
Conflict of interest statement
The author Giuseppe Celenza received a grant from Biolabo S.A.S. for research activities not related to the subject of this study. The other authors have no conflict of interest to disclose.
Figures

References
-
- Medical Laboratories—Requirements for Quality and Competence. ISO; Geneva, Switzerland: 2012. [(accessed on 13 December 2022)]. Available online: https://www.iso.org/standard/56115.html.
-
- Antonelli G., Sciacovelli L., Aita A., Bozzato D., Plebani M. The Pathway for Introducing Novel Examination Procedures in Routine Practice in Accordance with ISO 15189:2012: 17-Hydroxy Progesterone, Dehydroepiandrosterone Sulphate and Vitamin D as Examples. Ann. Clin. Biochem. 2019;56:548–555. doi: 10.1177/0004563219835582. - DOI - PubMed
-
- Antonelli G., Padoan A., Aita A., Sciacovelli L., Plebani M. Verification of Examination Procedures in Clinical Laboratory for Imprecision, Trueness and Diagnostic Accuracy According to ISO 15189:2012: A Pragmatic Approach. Clin. Chem. Lab. Med. 2017;55:1501–1508. doi: 10.1515/cclm-2016-0894. - DOI - PubMed
LinkOut - more resources
Full Text Sources

