Mitochondrial Unfolded Protein Response and Integrated Stress Response as Promising Therapeutic Targets for Mitochondrial Diseases
- PMID: 36611815
- PMCID: PMC9818186
- DOI: 10.3390/cells12010020
Mitochondrial Unfolded Protein Response and Integrated Stress Response as Promising Therapeutic Targets for Mitochondrial Diseases
Abstract
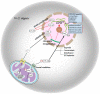
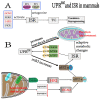
The development and application of high-throughput omics technologies have enabled a more in-depth understanding of mitochondrial biosynthesis metabolism and the pathogenesis of mitochondrial diseases. In accordance with this, a host of new treatments for mitochondrial disease are emerging. As an essential pathway in maintaining mitochondrial proteostasis, the mitochondrial unfolded protein response (UPRmt) is not only of considerable significance for mitochondrial substance metabolism but also plays a fundamental role in the development of mitochondrial diseases. Furthermore, in mammals, the integrated stress response (ISR) and UPRmt are strongly coupled, functioning together to maintain mitochondrial function. Therefore, ISR and UPRmt show great application prospects in the treatment of mitochondrial diseases. In this review, we provide an overview of the molecular mechanisms of ISR and UPRmt and focus on them as potential targets for mitochondrial disease therapy.
Keywords: integrated stress response; mitochondrial diseases; mitochondrial function; mitochondrial unfolded protein response.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

