Human Neutrophils Generate Extracellular Vesicles That Modulate Their Functional Responses
- PMID: 36611930
- PMCID: PMC9818892
- DOI: 10.3390/cells12010136
Human Neutrophils Generate Extracellular Vesicles That Modulate Their Functional Responses
Abstract
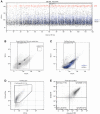
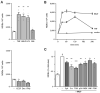
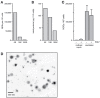
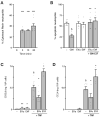
Neutrophils influence innate and adaptive immunity by releasing various cytokines and chemokines, by generating neutrophil extracellular traps (NETs), and by modulating their own survival. Neutrophils also produce extracellular vesicles (EVs) termed ectosomes, which influence the function of other immune cells. Here, we studied neutrophil-derived ectosomes (NDEs) and whether they can modulate autologous neutrophil responses. We first characterized EV production by neutrophils, following MISEV 2018 guidelines to facilitate comparisons with other studies. We found that such EVs are principally NDEs, that they are rapidly released in response to several (but not all) physiological stimuli, and that a number of signaling pathways are involved in the induction of this response. When co-incubated with autologous neutrophils, NDE constituents were rapidly incorporated into recipient cells and this triggered and/or modulated neutrophil responses. The pro-survival effect of GM-CSF, G-CSF, IFNγ, and dexamethasone was reversed; CXCL8 and NET formation were induced in otherwise unstimulated neutrophils; the induction of inflammatory chemokines by TNFα was modulated depending on the activation state of the NDEs' parent cells; and inducible NET generation was attenuated. Our data show that NDE generation modulates neutrophil responses in an autocrine and paracrine manner, and indicate that this probably represents an important aspect of how neutrophils shape their environment and cellular interactions.
Keywords: NETs; apoptosis; autocrine; chemokines; extracellular vesicles; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. PPMcD is also employed as Executive Director, Research at Insmed Inc. Insmed was, however, not involved in this work; did not fund it; and does not endorse it, implicitly or otherwise.
Figures


References
-
- Metschnikoff E. Immunität bei Infektionskrankheiten. Verlag Gustav Fischer; Jena, Germany: 1902.
-
- Khoyratty T.E., Ai Z., Ballesteros I., Eames H.L., Mathie S., Martín-Salamanca S., Wang L., Hemmings A., Willemsen N., von Werz V., et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat. Immunol. 2021;22:1093–1106. doi: 10.1038/s41590-021-00968-4. - DOI - PMC - PubMed
-
- Veglia F., Hashimoto A., Dweep H., Sanseviero E., De Leo A., Tcyganov E., Kossenkov A., Mulligan C., Nam B., Masters G., et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021;218:e20201803. doi: 10.1084/jem.20201803. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

