Preclinical Studies of Posttraumatic Headache and the Potential Therapeutics
- PMID: 36611947
- PMCID: PMC9818317
- DOI: 10.3390/cells12010155
Preclinical Studies of Posttraumatic Headache and the Potential Therapeutics
Abstract
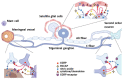
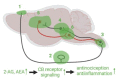
Posttraumatic headache (PTH) attributed to traumatic brain injury (TBI) is a secondary headache developed within 7 days after head injury, and in a substantial number of patients PTH becomes chronic and lasts for more than 3 months. Current medications are almost entirely relied on the treatment of primary headache such as migraine, due to its migraine-like phenotype and the limited understanding on the PTH pathogenic mechanisms. To this end, increasing preclinical studies have been conducted in the last decade. We focus in this review on the trigeminovascular system from the animal studies since it provides the primary nociceptive sensory afferents innervating the head and face region, and the pathological changes in the trigeminal pathway are thought to play a key role in the development of PTH. In addition to the pathologies, PTH-like behaviors induced by TBI and further exacerbated by nitroglycerin, a general headache inducer through vasodilation are reviewed. We will overview the current pharmacotherapies including calcitonin gene-related peptide (CGRP) monoclonal antibody and sumatriptan in the PTH animal models. Given that modulation of the endocannabinoid (eCB) system has been well-documented in the treatment of migraine and TBI, the therapeutic potential of eCB in PTH will also be discussed.
Keywords: CGRP; endocannabinoids; migraine; posttraumatic headache; traumatic brain injury; trigeminovascular system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inhibition of 2-AG hydrolysis alleviates posttraumatic headache attributed to mild traumatic brain injury.J Headache Pain. 2024 Jul 16;25(1):115. doi: 10.1186/s10194-024-01817-z. J Headache Pain. 2024. PMID: 39014318 Free PMC article.
-
CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice.Cephalalgia. 2019 Dec;39(14):1762-1775. doi: 10.1177/0333102419877662. Epub 2019 Sep 24. Cephalalgia. 2019. PMID: 31550910
-
Enhanced post-traumatic headache-like behaviors and diminished contribution of peripheral CGRP in female rats following a mild closed head injury.Cephalalgia. 2020 Jun;40(7):748-760. doi: 10.1177/0333102420907597. Epub 2020 Feb 20. Cephalalgia. 2020. PMID: 32077327 Free PMC article.
-
Onabotulinumtoxin A for the Treatment of Post-Traumatic Headache: Is It Better than Anti-CGRP Antibodies?Toxins (Basel). 2024 Oct 2;16(10):427. doi: 10.3390/toxins16100427. Toxins (Basel). 2024. PMID: 39453203 Free PMC article. Review.
-
Post-traumatic headache: epidemiology and pathophysiological insights.Nat Rev Neurol. 2019 Oct;15(10):607-617. doi: 10.1038/s41582-019-0243-8. Epub 2019 Sep 16. Nat Rev Neurol. 2019. PMID: 31527806 Review.
Cited by
-
Substrate-selective COX-2 inhibition by IMMA attenuates posttraumatic headache via endocannabinoid modulation and neuroinflammatory suppression.J Headache Pain. 2025 Aug 12;26(1):183. doi: 10.1186/s10194-025-02116-x. J Headache Pain. 2025. PMID: 40797173 Free PMC article.
-
Exploring cerebral structural and functional abnormalities in a mouse model of post-traumatic headache induced by mild traumatic brain injury.Zool Res. 2024 May 18;45(3):648-662. doi: 10.24272/j.issn.2095-8137.2023.323. Zool Res. 2024. PMID: 38766747 Free PMC article.
-
Biomarkers and Endophenotypes of Post-traumatic Headaches.Curr Pain Headache Rep. 2024 Dec;28(12):1185-1193. doi: 10.1007/s11916-024-01255-1. Epub 2024 Aug 13. Curr Pain Headache Rep. 2024. PMID: 39136870 Review.
-
Inhibition of 2-AG hydrolysis alleviates posttraumatic headache attributed to mild traumatic brain injury.J Headache Pain. 2024 Jul 16;25(1):115. doi: 10.1186/s10194-024-01817-z. J Headache Pain. 2024. PMID: 39014318 Free PMC article.
-
Conservative Management of Acute Sports-Related Concussions: A Narrative Review.Healthcare (Basel). 2024 Jan 23;12(3):289. doi: 10.3390/healthcare12030289. Healthcare (Basel). 2024. PMID: 38338173 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

