Utility of the Cerebral Organoid Glioma 'GLICO' Model for Screening Applications
- PMID: 36611949
- PMCID: PMC9818141
- DOI: 10.3390/cells12010153
Utility of the Cerebral Organoid Glioma 'GLICO' Model for Screening Applications
Abstract
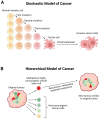
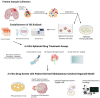
Glioblastoma, a grade IV astrocytoma, is regarded as the most aggressive primary brain tumour with an overall median survival of 16.0 months following the standard treatment regimen of surgical resection, followed by radiotherapy and chemotherapy with temozolomide. Despite such intensive treatment, the tumour almost invariably recurs. This poor prognosis has most commonly been attributed to the initiation, propagation, and differentiation of cancer stem cells. Despite the unprecedented advances in biomedical research over the last decade, the current in vitro models are limited at preserving the inter- and intra-tumoural heterogeneity of primary tumours. The ability to understand and manipulate complex cancers such as glioblastoma requires disease models to be clinically and translationally relevant and encompass the cellular heterogeneity of such cancers. Therefore, brain cancer research models need to aim to recapitulate glioblastoma stem cell function, whilst remaining amenable for analysis. Fortunately, the recent development of 3D cultures has overcome some of these challenges, and cerebral organoids are emerging as cutting-edge tools in glioblastoma research. The opportunity to generate cerebral organoids via induced pluripotent stem cells, and to perform co-cultures with patient-derived cancer stem cells (GLICO model), has enabled the analysis of cancer development in a context that better mimics brain tissue architecture. In this article, we review the recent literature on the use of patient-derived glioblastoma organoid models and their applicability for drug screening, as well as provide a potential workflow for screening using the GLICO model. The proposed workflow is practical for use in most laboratories with accessible materials and equipment, a good first pass, and no animal work required. This workflow is also amenable for analysis, with separate measures of invasion, growth, and viability.
Keywords: cancer stem cells; cerebral organoids; drug screening; glioblastoma; glioblastoma organoids; glioblastoma spheroids; glioblastoma stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Modeling Patient-Derived Glioblastoma with Cerebral Organoids.Cell Rep. 2019 Mar 19;26(12):3203-3211.e5. doi: 10.1016/j.celrep.2019.02.063. Cell Rep. 2019. PMID: 30893594 Free PMC article.
-
The Organoid Era Permits the Development of New Applications to Study Glioblastoma.Cancers (Basel). 2020 Nov 9;12(11):3303. doi: 10.3390/cancers12113303. Cancers (Basel). 2020. PMID: 33182346 Free PMC article. Review.
-
Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology.Acta Neuropathol. 2020 Dec;140(6):919-949. doi: 10.1007/s00401-020-02226-7. Epub 2020 Oct 3. Acta Neuropathol. 2020. PMID: 33009951 Free PMC article.
-
Three-dimensional model of glioblastoma by co-culturing tumor stem cells with human brain organoids.Biol Open. 2021 Feb 22;10(2):bio056416. doi: 10.1242/bio.056416. Biol Open. 2021. PMID: 33619017 Free PMC article.
-
Cerebral organoids: emerging ex vivo humanoid models of glioblastoma.Acta Neuropathol Commun. 2020 Dec 1;8(1):209. doi: 10.1186/s40478-020-01077-3. Acta Neuropathol Commun. 2020. PMID: 33261657 Free PMC article. Review.
Cited by
-
Development of glioblastoma organoids and their applications in personalized therapy.Cancer Biol Med. 2023 Jun 5;20(5):353-68. doi: 10.20892/j.issn.2095-3941.2023.0061. Cancer Biol Med. 2023. PMID: 37283493 Free PMC article. Review.
-
Functional and Molecular Heterogeneity in Glioma Stem Cells Derived from Multiregional Sampling.Cancers (Basel). 2023 Dec 13;15(24):5826. doi: 10.3390/cancers15245826. Cancers (Basel). 2023. PMID: 38136371 Free PMC article.
-
Stem cell modeling of nervous system tumors.Dis Model Mech. 2024 Feb 1;17(2):dmm050533. doi: 10.1242/dmm.050533. Epub 2024 Feb 14. Dis Model Mech. 2024. PMID: 38353122 Free PMC article. Review.
-
The tumour microenvironment, treatment resistance and recurrence in glioblastoma.J Transl Med. 2024 Jun 6;22(1):540. doi: 10.1186/s12967-024-05301-9. J Transl Med. 2024. PMID: 38844944 Free PMC article. Review.
-
Glioblastoma Biology, Genetics and Possible Therapies.Cells. 2023 Aug 14;12(16):2063. doi: 10.3390/cells12162063. Cells. 2023. PMID: 37626873 Free PMC article.
References
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

