Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health
- PMID: 36611977
- PMCID: PMC9818925
- DOI: 10.3390/cells12010184
Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health
Abstract
Immune cells and commensal microbes in the human intestine constantly communicate with and react to each other in a stable environment in order to maintain healthy immune activities. Immune system-microbiota cross-talk relies on a complex network of pathways that sustain the balance between immune tolerance and immunogenicity. Probiotic bacteria can interact and stimulate intestinal immune cells and commensal microflora to modulate specific immune functions and immune homeostasis. Growing evidence shows that probiotic bacteria present important health-promoting and immunomodulatory properties. Thus, the use of probiotics might represent a promising approach for improving immune system activities. So far, few studies have been reported on the beneficial immune modulatory effect of probiotics. However, many others, which are mainly focused on their metabolic/nutritional properties, have been published. Therefore, the mechanisms behind the interaction between host immune cells and probiotics have only been partially described. The present review aims to collect and summarize the most recent scientific results and the resulting implications of how probiotic bacteria and immune cells interact to improve immune functions. Hence, a description of the currently known immunomodulatory mechanisms of probiotic bacteria in improving the host immune system is provided.
Keywords: beneficial microbes; human health; immune cells; immune modulation; immune response; immune system; microbial modulation effects; microbiome; microbiota; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
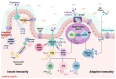
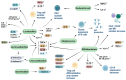
References
-
- Sharifi-Rad J., Rodrigues C.F., Stojanović-Radić Z., Dimitrijević M., Aleksić A., Neffe-Skocińska K., Zielińska D., Kołożyn-Krajewska D., Salehi B., Prabu S.M., et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina. 2020;56:433. doi: 10.3390/medicina56090433. - DOI - PMC - PubMed
-
- Chinda D., Takada T., Mikami T., Shimizu K., Oana K., Arai T., Akitaya K., Sakuraba H., Katto M., Nagara Y., et al. Spatial distribution of live gut microbiota and bile acid metabolism in various parts of human large intestine. Sci. Rep. 2022;12:1–18. doi: 10.1038/s41598-022-07594-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

