Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis
- PMID: 36611982
- PMCID: PMC9818313
- DOI: 10.3390/cells12010189
Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis
Abstract
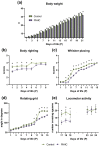
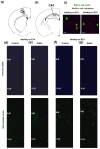
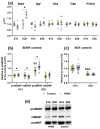
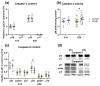
Maternal hyperhomocysteinemia causes the disruption of placental blood flow and can lead to serious disturbances in the formation of the offspring's brain. In the present study, the effects of prenatal hyperhomocysteinemia (PHHC) on the neuronal migration, neural tissue maturation, and the expression of signaling molecules in the rat fetal brain were described. Maternal hyperhomocysteinemia was induced in female rats by per os administration of 0.15% aqueous methionine solution in the period of days 4-21 of pregnancy. Behavioral tests revealed a delay in PHHC male pups maturing. Ultrastructure of both cortical and hippocampus tissue demonstrated the features of the developmental delay. PHHC was shown to disturb both generation and radial migration of neuroblasts into the cortical plate. Elevated Bdnf expression, together with changes in proBDNF/mBDNF balance, might affect neuronal cell viability, positioning, and maturation in PHHC pups. Reduced Kdr gene expression and the content of SEMA3E might lead to impaired brain development. In the brain tissue of E20 PHHC fetuses, the content of the procaspase-8 was decreased, and the activity level of the caspase-3 was increased; this may indicate the development of apoptosis. PHHC disturbs the mechanisms of early brain development leading to a delay in brain tissue maturation and formation of the motor reaction of pups.
Keywords: caspase; electron microscopy; hippocampus; homocysteine; matrix metalloproteinase; neocortex; neurodegeneration; neurotrophins; rat; semaphorin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Neurotrophins of the Fetal Brain and Placenta in Prenatal Hyperhomocysteinemia.Biochemistry (Mosc). 2020 Feb;85(2):213-223. doi: 10.1134/S000629792002008X. Biochemistry (Mosc). 2020. PMID: 32093597
-
Prenatal Hyperhomocysteinemia Induces Glial Activation and Alters Neuroinflammatory Marker Expression in Infant Rat Hippocampus.Cells. 2021 Jun 18;10(6):1536. doi: 10.3390/cells10061536. Cells. 2021. PMID: 34207057 Free PMC article.
-
Maternal Hyperhomocysteinemia Induces Neuroinflammation and Neuronal Death in the Rat Offspring Cortex.Neurotox Res. 2020 Aug;38(2):408-420. doi: 10.1007/s12640-020-00233-w. Epub 2020 Jun 5. Neurotox Res. 2020. PMID: 32504390
-
Prenatal Stress in Maternal Hyperhomocysteinemia: Impairments in the Fetal Nervous System Development and Placental Function.Biochemistry (Mosc). 2021 Jun;86(6):716-728. doi: 10.1134/S0006297921060092. Biochemistry (Mosc). 2021. PMID: 34225594 Review.
-
Epigenetic Mechanisms Involved in the Effects of Maternal Hyperhomocysteinemia on the Functional State of Placenta and Nervous System Plasticity in the Offspring.Biochemistry (Mosc). 2023 Apr;88(4):435-456. doi: 10.1134/S0006297923040016. Biochemistry (Mosc). 2023. PMID: 37080931 Review.
Cited by
-
Diet-induced hyperhomocysteinemia causes sex-dependent deficiencies in offspring musculature and brain function.Front Cell Dev Biol. 2024 Mar 15;12:1322844. doi: 10.3389/fcell.2024.1322844. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38559811 Free PMC article.
-
Prenatal Hyperhomocysteinemia Leads to Synaptic Dysfunction and Structural Alterations in the CA1 Hippocampus of Rats.Biomolecules. 2025 Feb 19;15(2):305. doi: 10.3390/biom15020305. Biomolecules. 2025. PMID: 40001608 Free PMC article.
-
The Role of Purinergic Mechanisms in the Excitability of Trigeminal Afferents of Rats with Prenatal Hyperhomocysteinemia.Biomolecules. 2025 Mar 15;15(3):419. doi: 10.3390/biom15030419. Biomolecules. 2025. PMID: 40149955 Free PMC article.
References
-
- Blaise S.A., Nedelec E., Schroeder H., Alberto J.M., Bossenmeyer-Pourie C., Gueant J.L., Daval J.L. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am. J. Pathol. 2007;170:667–679. doi: 10.2353/ajpath.2007.060339. - DOI - PMC - PubMed
-
- Schweinberger B.M., Rodrigues A.F., Dos Santos T.M., Rohden F., Barbosa S., da Luz Soster P.R., Partata W.A., Faccioni-Heuser M.C., Wyse A.T.S. Methionine administration in pregnant rats causes memory deficit in the offspring and alters ultrastructure in brain tissue. Neurotox. Res. 2018;33:239–246. doi: 10.1007/s12640-017-9830-x. - DOI - PubMed
-
- Shcherbitskaia A.D., Vasilev D.S., Milyutina Y.P., Tumanova N.L., Mikhel A.V., Zalozniaia I.V., Arutjunyan A.V. Prenatal Hyperhomocysteinemia Induces Glial Activation and Alters Neuroinflammatory Marker Expression in Infant Rat Hippocampus. Cells. 2021;10:1536. doi: 10.3390/cells10061536. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

