The Lymphatic Endothelium in the Context of Radioimmuno-Oncology
- PMID: 36612017
- PMCID: PMC9817924
- DOI: 10.3390/cancers15010021
The Lymphatic Endothelium in the Context of Radioimmuno-Oncology
Abstract
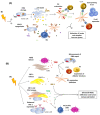
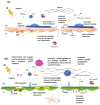
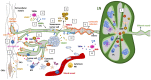
The study of lymphatic tumor vasculature has been gaining interest in the context of cancer immunotherapy. These vessels constitute conduits for immune cells' transit toward the lymph nodes, and they endow tumors with routes to metastasize to the lymph nodes and, from them, toward distant sites. In addition, this vasculature participates in the modulation of the immune response directly through the interaction with tumor-infiltrating leukocytes and indirectly through the secretion of cytokines and chemokines that attract leukocytes and tumor cells. Radiotherapy constitutes the therapeutic option for more than 50% of solid tumors. Besides impacting transformed cells, RT affects stromal cells such as endothelial and immune cells. Mature lymphatic endothelial cells are resistant to RT, but we do not know to what extent RT may affect tumor-aberrant lymphatics. RT compromises lymphatic integrity and functionality, and it is a risk factor to the onset of lymphedema, a condition characterized by deficient lymphatic drainage and compromised tissue homeostasis. This review aims to provide evidence of RT's effects on tumor vessels, particularly on lymphatic endothelial cell physiology and immune properties. We will also explore the therapeutic options available so far to modulate signaling through lymphatic endothelial cell receptors and their repercussions on tumor immune cells in the context of cancer. There is a need for careful consideration of the RT dosage to come to terms with the participation of the lymphatic vasculature in anti-tumor response. Here, we provide new approaches to enhance the contribution of the lymphatic endothelium to radioimmuno-oncology.
Keywords: lymphatic system; lymphedema; radioimmuno-oncology; radioresistance; vasculature.
Conflict of interest statement
M.E.R.-R reports receiving research funding from Roche and Highlight Therapeutics. She also has received speaker’s bureau honoraria from BMS and ROCHE. L.S. and A.R. declare no competing interests.
Figures
References
-
- Applegate K.E., Rühm W., Wojcik A., Bourguignon M., Brenner A., Hamasaki K., Imai T., Imaizumi M., Imaoka T., Kakinuma S., et al. Individual response of humans to ionising radiation: Governing factors and importance for radiological protection. Radiat. Environ. Biophys. 2020;59:185–209. doi: 10.1007/s00411-020-00837-y. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

