Cancer-Associated Fibroblast Diversity Shapes Tumor Metabolism in Pancreatic Cancer
- PMID: 36612058
- PMCID: PMC9817728
- DOI: 10.3390/cancers15010061
Cancer-Associated Fibroblast Diversity Shapes Tumor Metabolism in Pancreatic Cancer
Abstract
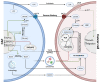
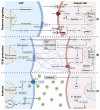
Despite extensive research, the 5-year survival rate of pancreatic cancer (PDAC) patients remains at only 9%. Patients often show poor treatment response, due partly to a highly complex tumor microenvironment (TME). Cancer-associated fibroblast (CAF) heterogeneity is characteristic of the pancreatic TME, where several CAF subpopulations have been identified, such as myofibroblastic CAFs (myCAFs), inflammatory CAFs (iCAFs), and antigen presenting CAFs (apCAFs). In PDAC, cancer cells continuously adapt their metabolism (metabolic switch) to environmental changes in pH, oxygenation, and nutrient availability. Recent advances show that these environmental alterations are all heavily driven by stromal CAFs. CAFs and cancer cells exchange cytokines and metabolites, engaging in a tight bidirectional crosstalk, which promotes tumor aggressiveness and allows constant adaptation to external stress, such as chemotherapy. In this review, we summarize CAF diversity and CAF-mediated metabolic rewiring, in a PDAC-specific context. First, we recapitulate the most recently identified CAF subtypes, focusing on the cell of origin, activation mechanism, species-dependent markers, and functions. Next, we describe in detail the metabolic crosstalk between CAFs and tumor cells. Additionally, we elucidate how CAF-driven paracrine signaling, desmoplasia, and acidosis orchestrate cancer cell metabolism. Finally, we highlight how the CAF/cancer cell crosstalk could pave the way for new therapeutic strategies.
Keywords: CAF; PDAC; acidosis; cancer-associated fibroblast; desmoplasia; hypoxia; metabolism; pancreatic cancer; paracrine signaling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Orth M., Metzger P., Gerum S., Mayerle J., Schneider G., Belka C., Schnurr M., Lauber K. Pancreatic Ductal Adenocarcinoma: Biological Hallmarks, Current Status, and Future Perspectives of Combined Modality Treatment Approaches. Radiat. Oncol. 2019;14:141. doi: 10.1186/s13014-019-1345-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

