The Effect of Oxidative Phosphorylation on Cancer Drug Resistance
- PMID: 36612059
- PMCID: PMC9817696
- DOI: 10.3390/cancers15010062
The Effect of Oxidative Phosphorylation on Cancer Drug Resistance
Abstract
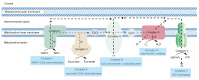
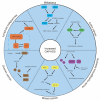
Recent studies have shown that oxidative phosphorylation (OXPHOS) is a target for the effective attenuation of cancer drug resistance. OXPHOS inhibitors can improve treatment responses to anticancer therapy in certain cancers, such as melanomas, lymphomas, colon cancers, leukemias and pancreatic ductal adenocarcinoma (PDAC). However, the effect of OXPHOS on cancer drug resistance is complex and associated with cell types in the tumor microenvironment (TME). Cancer cells universally promote OXPHOS activity through the activation of various signaling pathways, and this activity is required for resistance to cancer therapy. Resistant cancer cells are prevalent among cancer stem cells (CSCs), for which the main metabolic phenotype is increased OXPHOS. CSCs depend on OXPHOS to survive targeting by anticancer drugs and can be selectively eradicated by OXPHOS inhibitors. In contrast to that in cancer cells, mitochondrial OXPHOS is significantly downregulated in tumor-infiltrating T cells, impairing antitumor immunity. In this review, we summarize novel research showing the effect of OXPHOS on cancer drug resistance, thereby explaining how this metabolic process plays a dual role in cancer progression. We highlight the underlying mechanisms of metabolic reprogramming in cancer cells, as it is vital for discovering new drug targets.
Keywords: cancer immunity; glycolysis; metabolism; oxidative phosphorylation; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oxidative Phosphorylation as an Emerging Target in Cancer Therapy.Clin Cancer Res. 2018 Jun 1;24(11):2482-2490. doi: 10.1158/1078-0432.CCR-17-3070. Epub 2018 Feb 2. Clin Cancer Res. 2018. PMID: 29420223 Review.
-
Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors.Redox Biol. 2019 Jul;25:101073. doi: 10.1016/j.redox.2018.101073. Epub 2018 Dec 13. Redox Biol. 2019. PMID: 30594485 Free PMC article. Review.
-
Targeting cancer stem cell OXPHOS with tailored ruthenium complexes as a new anti-cancer strategy.J Exp Clin Cancer Res. 2024 Jan 27;43(1):33. doi: 10.1186/s13046-023-02931-7. J Exp Clin Cancer Res. 2024. PMID: 38281027 Free PMC article.
-
Targeting Mitochondrial Complex I Overcomes Chemoresistance in High OXPHOS Pancreatic Cancer.Cell Rep Med. 2020 Nov 17;1(8):100143. doi: 10.1016/j.xcrm.2020.100143. eCollection 2020 Nov 17. Cell Rep Med. 2020. PMID: 33294863 Free PMC article.
-
Therapeutic Targeting of Tumor Cells and Tumor Immune Microenvironment Vulnerabilities.Front Oncol. 2022 Jun 8;12:816504. doi: 10.3389/fonc.2022.816504. eCollection 2022. Front Oncol. 2022. PMID: 35756631 Free PMC article. Review.
Cited by
-
Fluorescence microscopy imaging of mitochondrial metabolism in cancer cells.Front Oncol. 2023 Jun 22;13:1152553. doi: 10.3389/fonc.2023.1152553. eCollection 2023. Front Oncol. 2023. PMID: 37427141 Free PMC article. Review.
-
Gravitational forces and matrix stiffness modulate the invasiveness of breast cancer cells in bioprinted spheroids.Mater Today Bio. 2025 Mar 4;31:101640. doi: 10.1016/j.mtbio.2025.101640. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124331 Free PMC article.
-
Identification of FLYWCH1 as a regulator of platinum-resistance in epithelial ovarian cancer.NAR Cancer. 2025 Apr 4;7(2):zcaf012. doi: 10.1093/narcan/zcaf012. eCollection 2025 Jun. NAR Cancer. 2025. PMID: 40191655 Free PMC article.
-
OXPHOS-targeting drugs in oncology: new perspectives.Expert Opin Ther Targets. 2023 Jul-Dec;27(10):939-952. doi: 10.1080/14728222.2023.2261631. Epub 2023 Oct 30. Expert Opin Ther Targets. 2023. PMID: 37736880 Free PMC article. Review.
-
Metabolism-focused CRISPR screen unveils mitochondrial pyruvate carrier 1 as a critical driver for PARP inhibitor resistance in lung cancer.Mol Carcinog. 2024 Jun;63(6):1024-1037. doi: 10.1002/mc.23705. Epub 2024 Feb 27. Mol Carcinog. 2024. PMID: 38411275 Free PMC article.
References
-
- Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12:1131–1137. doi: 10.1007/BF01504608. - DOI
-
- Gonçalves A.C., Richiardone E., Jorge J., Polónia B., Xavier C.P.R., Salaroglio I.C., Riganti C., Vasconcelos M.H., Corbet C., Sarmento-Ribeiro A.B. Impact of cancer metabolism on therapy resistance—Clinical implications. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2021;59:100797. doi: 10.1016/j.drup.2021.100797. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

