Mechanisms and Strategies to Overcome PD-1/PD-L1 Blockade Resistance in Triple-Negative Breast Cancer
- PMID: 36612100
- PMCID: PMC9817764
- DOI: 10.3390/cancers15010104
Mechanisms and Strategies to Overcome PD-1/PD-L1 Blockade Resistance in Triple-Negative Breast Cancer
Abstract
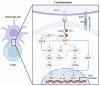
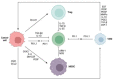
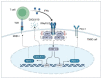
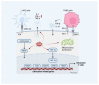
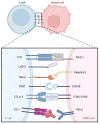
Triple-negative breast cancer (TNBC) is characterized by a high rate of systemic metastasis, insensitivity to conventional treatment and susceptibility to drug resistance, resulting in a poor patient prognosis. The immune checkpoint inhibitors (ICIs) represented by antibodies of programmed death receptor 1 (PD-1) and programmed death receptor ligand 1 (PD-L1) have provided new therapeutic options for TNBC. However, the efficacy of PD-1/PD-L1 blockade monotherapy is suboptimal immune response, which may be caused by reduced antigen presentation, immunosuppressive tumor microenvironment, interplay with other immune checkpoints and aberrant activation of oncological signaling in tumor cells. Therefore, to improve the sensitivity of TNBC to ICIs, suitable patients are selected based on reliable predictive markers and treated with a combination of ICIs with other therapies such as chemotherapy, radiotherapy, targeted therapy, oncologic virus and neoantigen-based therapies. This review discusses the current mechanisms underlying the resistance of TNBC to PD-1/PD-L1 inhibitors, the potential biomarkers for predicting the efficacy of anti-PD-1/PD-L1 immunotherapy and recent advances in the combination therapies to increase response rates, the depth of remission and the durability of the benefit of TNBC to ICIs.
Keywords: PD-1/PD-L1; combination therapy; immune checkpoint inhibitor; resistance; triple-negative breast cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

