Endometriosis Stem Cells as a Possible Main Target for Carcinogenesis of Endometriosis-Associated Ovarian Cancer (EAOC)
- PMID: 36612107
- PMCID: PMC9817684
- DOI: 10.3390/cancers15010111
Endometriosis Stem Cells as a Possible Main Target for Carcinogenesis of Endometriosis-Associated Ovarian Cancer (EAOC)
Abstract
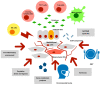
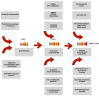
Endometriosis is a serious recurrent disease impairing the quality of life and fertility, and being a risk for some histologic types of ovarian cancer defined as endometriosis-associated ovarian cancers (EAOC). The presence of stem cells in the endometriotic foci could account for the proliferative, migrative and angiogenic activity of the lesions. Their phenotype and sources have been described. The similarly disturbed expression of several genes, miRNAs, galectins and chaperones has been observed both in endometriotic lesions and in ovarian or endometrial cancer. The importance of stem cells for nascence and sustain of malignant tumors is commonly appreciated. Although the proposed mechanisms promoting carcinogenesis leading from endometriosis into the EAOC are not completely known, they have been discussed in several articles. However, the role of endometriosis stem cells (ESCs) has not been discussed in this context. Here, we postulate that ESCs may be a main target for the carcinogenesis of EAOC and present the possible sequence of events resulting finally in the development of EAOC.
Keywords: endometriosis; endometriosis stem cells; endometriosis-associated ovarian cancer; ovarian cancer; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

