Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer
- PMID: 36612253
- PMCID: PMC9818718
- DOI: 10.3390/cancers15010258
Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer
Abstract
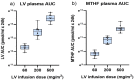
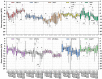
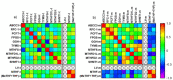
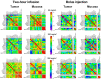
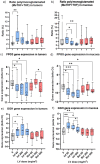
The combination of 5-fluorouracil (5-FU) and leucovorin (LV) forms the chemotherapy backbone for patients with colorectal cancer. However, the LV administration is often standardized and not based on robust scientific data. To address these issues, a randomized pharmacokinetics study was performed in patients with colon cancer. Thirty patients were enrolled, receiving 60, 200 or 500 mg/m2 LV as a single two-hour infusion. Blood, tumor, mucosa, and resection margin biopsies were collected. Folate concentrations were analyzed with LC-MS/MS and gene expression with qPCR. Data from a previous study where patients received LV as bolus injections were used as comparison. Saturation of methylenetetrahydrofolate (MeTHF) and tetrahydrofolate (THF) levels was seen after two-hour infusion and polyglutamated MeTHF + THF levels in tumors decreased with increasing LV dosage. The decrease was associated with decreased FPGS and increased GGH expression, which was not observed after LV bolus injection. In the bolus group, results indicate activation of a metabolic switch possibly promoting TYMS inhibition in response to 5-FU. Different metabolic mechanisms appear to be induced when LV is administered as infusion and bolus injection. Since maximal inhibition of TYMS by the 5-FU metabolite 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) requires excess polyglutamated MeTHF, the results point in favor of the bolus regimen.
Keywords: LC-MS/MS; bolus injection; colon cancer; gene expression; leucovorin; methylenetetrahydrofolate; pharmacokinetics; two-hour infusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schmoll H.J., Van Cutsem E., Stein A., Valentini V., Glimelius B., Haustermans K., Nordlinger B., van de Velde C.J., Balmana J., Regula J., et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. - DOI - PubMed
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Piedbois P., Buyse M., Rustum Y., Machover D., Erlichman C., Carlson R.W., Valone F., Labianca R., Doroshow J.H., Petrelli N. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: Evidence in terms of response rate by the advanced colorectal cancer meta-analysis project. J. Clin. Oncol. 1992;10:896–903. doi: 10.1200/JCO.1992.10.6.896. - DOI - PubMed
Grants and funding
- CAN 2015/499, CAN 2018/620, CAN 201025PjF/Swedish Cancer Society
- 2016:70/King Gustav V Jubilee Clinic Foundation for Cancer Research
- ALFGBG-426941, ALFGBG-586631, ALFGBG-723361, ALFGBG-788901, ALFGBG-966007/The Swedish State under the LUA/ALF agreement
- SLS-689001/The Swedish Society of Medicine
- FB17-82/The Assar Gabrielssons Foundation
LinkOut - more resources
Full Text Sources
Miscellaneous

