Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review
- PMID: 36612259
- PMCID: PMC9818311
- DOI: 10.3390/cancers15010264
Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review
Abstract
Objective: To summarize the function and efficacy of immunotherapy as an adjunctive therapy in the treatment of advanced chordoma.
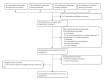
Methods: Literature search was conducted by two reviewers independently. Case reports, case series and clinical trials of immunotherapy for chordoma were retrieved systematically from Pubmed, Web of Science, Scoupus and Cochrane Library. Clinical outcome data extracted from the literature included median progression-free survival (PFS), median overall survival (OS), clinical responses and adverse events (AEs).
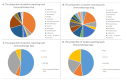
Results: All studies were published between 2015 and 2022. Twenty-two eligible studies were selected for systemic review. PD-1/PD-L1 immune checkpoint inhibitors (ICIs) were the most common used immunotherapy agents in chordoma, among which Pembrolizumab was the most frequently prescribed. CTLA-4 antibody was only used as combination therapy in chordoma. Dose Limiting Toxicity (DLT) was not observed in any vaccine targeting brachyury, and injection site response was the most frequent AV. The response evaluation criteria in solid tumors (RECIST) were the most generally used evaluation standard in chordoma immunotherapy, and none of the included studies employed the Choi criteria.
Conclusions: No clinical data have demonstrated that CTLA-4 ICIs combined with PD-1/PD-L1 ICIs is more effective than ICIs monotherapy in treating chordoma, and ICIs in combination with other therapies exhibit more toxicity than monotherapy. PD-1/PD-L1 ICIs monotherapy is recommended as an immunotherapy in patients with advanced chordoma, which may even benefit PD-L1-negative patients. The brachyury vaccine has shown good safety in chordoma patients, and future clinical trials should focus on how to improve its therapeutic efficacy. The use of immunomodulatory agents is a promising therapeutic option, though additional clinical trials are required to evaluate their safety and effectiveness. RECIST does not seem to be an appropriate standard for assessing medications of intratumoral immunotherapy.
Keywords: chordoma; combination; immune checkpoint inhibitor; immunotherapy; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization . WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours. Volume 5 World Health Organization; Geneva, Switzerland: 2013.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

