PSMA-RLT in Patients with Metastatic Hormone-Sensitive Prostate Cancer: A Retrospective Study
- PMID: 36612293
- PMCID: PMC9818570
- DOI: 10.3390/cancers15010297
PSMA-RLT in Patients with Metastatic Hormone-Sensitive Prostate Cancer: A Retrospective Study
Abstract
Background: Prostate-specific membrane antigen (PSMA)-directed radioligand therapy (RLT) is a novel treatment for patients with castration-resistant prostate cancer (CRPC). Given the mode of action, patients in an earlier disease stage, such as hormone-sensitive prostate cancer (HSPC), are also likely to benefit from [177Lu]Lu-PSMA- (177Lu-PSMA) or [225Ac]Ac-PSMA-radioligand treatment (225Ac-PSMA). In this retrospective study, we analyzed the safety and efficacy of PSMA-RLT in early-stage and hormone-sensitive metastatic prostate cancer patients.
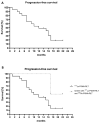
Methods: A retrospective study was performed in patients who received 177Lu-PSMA and/or 225Ac-PSMA with early-stage metastatic prostate cancer. The primary outcome parameter evaluated in this study was the progression-free survival (PFS) after PSMA-RLT and toxicity according to the Common Terminology Criteria for Adverse Events. Secondary outcome parameters were prostate-specific antigen (PSA) response and the date of onset of CRPC state.
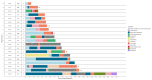
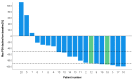
Results: In total, 20 patients were included of which 18 patients received 177Lu-PSMA radioligand and two patients received tandem treatment with both 177Lu-PSMA and 225Ac-PSMA radioligands. Patients received a median of 2 treatment cycles (range 1-6) and a median activity of 6.2 GBq 177Lu-PSMA per cycle (interquartile range (IQR) 5.2-7.4 GBq). PSMA-RLT was overall well-tolerated. The most common grade 1-2 side effects were xerostomia (n = 6) and fatigue (n = 8), which were only temporarily reported. One patient that received 225Ac-PSMA developed grade 3-4 bone marrow toxicity. The median PFS was 12 months (95% confidence interval (CI), 4.09-19.9 months). Seventeen (85%) patients had a ≥50% PSA response following PSMA-RLT. One patient developed CRPC 9 months following PSMA-RLT.
Conclusions: In this small cohort study, PSMA-RLT appeared safe and showed encouraging efficacy for (metastasized) early-stage and hormone-sensitive prostate cancer patients. Prospective studies are awaited and should include long-term follow-up.
Keywords: Actinium-225; Lutetium-177; PSMA radioligand therapy; early stage; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Morris M.J., De Bono J.S., Chi K.N., Fizazi K., Herrmann K., Rahbar K., Tagawa S.T., Nordquist L.T., Vaishampayan N., El-Haddad G., et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION) J. Clin. Oncol. 2021;39:LBA4. doi: 10.1200/JCO.2021.39.15_suppl.LBA4. - DOI
-
- Privé B.M., Peters S.M.B., Muselaers C.H.J., van Oort I.M., Janssen M.J.R., Sedelaar J.P.M., Konijnenberg M.W., Zámecnik P., Uijen M.J.M., Schilham M.G.M., et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: A prospective pilot study. Clin. Cancer Res. 2021;27:3595–3601. doi: 10.1158/1078-0432.CCR-20-4298. - DOI - PubMed
-
- Peters S.M.B., Privé B.M., de Bakker M., de Lange F., Jentzen W., Eek A., Muselaers C.H.J., Mehra N., Witjes J.A., Gotthardt M., et al. Intra-therapeutic dosimetry of [177Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur. J. Nucl. Med. Mol. Imaging. 2021;49:460–469. doi: 10.1007/s00259-021-05471-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

