Oligometastatic Prostate Cancer Treated with Metastasis-Directed Therapy Guided by Positron Emission Tomography: Does the Tracer Matter?
- PMID: 36612319
- PMCID: PMC9818332
- DOI: 10.3390/cancers15010323
Oligometastatic Prostate Cancer Treated with Metastasis-Directed Therapy Guided by Positron Emission Tomography: Does the Tracer Matter?
Abstract
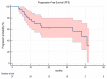
The superior diagnostic accuracy of [68Ga]Ga-prostate-specific membrane antigen-11 (PSMA) ([68Ga]Ga-PSMA-11) compared to [18F]F-Fluorocholine Positron Emission Tomography/Computed Tomography (PET/CT) in Prostate Cancer (PCa) is established. However, it is currently unclear if the added diagnostic accuracy actually translates into improved clinical outcomes in oligometastatic PCa patients treated with [68Ga]Ga-PSMA-11 PET-guided metastasis-directed therapy (MDT). The present study aimed to assess the impact of these two imaging techniques on Progression-Free Survival (PFS) in a real-world sample of oligometastatic PCa patients submitted to PET-guided MDT. Thirty-seven oligometastatic PCa patients treated with PET-guided MDT were retrospectively enrolled. MDT was guided by [18F]F-Fluorocholine PET/CT in eleven patients and by [68Ga]Ga-PSMA-11 PET/CT in twenty-six. Progression was defined as biochemical recurrence (BR), radiological progression at subsequent PET/CT imaging, clinical progression, androgen deprivation therapy initiation, or death. Clinical and imaging parameters were assessed as predictors of PFS. [18F]F-Fluorocholine PET-guided MDT was associated with significantly lower PFS compared to the [68Ga]Ga-PSMA-11 group (median PFS, mPFS 15.47 months, 95% CI: 4.13−38.00 vs. 40.93 months, 95% CI: 40.93−40.93, respectively; p < 0.05). Coherently, the radiotracer used for PET-guided MDT resulted in predictive PFS at the univariate analysis, as well as the castration-resistant status at the time of MDT and the PSA nadir after MDT. However, in the multivariate analysis, castration resistance and PSA nadir after MDT remained the sole independent predictors of PFS. In conclusion, in the present proof-of-concept study, [68Ga]Ga-PSMA-11 provided higher PFS rates than [18F]F-Fluorocholine imaging in oligometastatic PCa patients receiving PET-guided MDT. Although preliminary, this finding suggests that enlarging the “tip of the iceberg”, by detecting a major proportion of the submerged disease thanks to next-generation imaging may favourably impact the oncological outcome of oligometastatic PCa treated with MDT.
Keywords: PET/CT; SBRT; [18F]F-Fluorocholine; [68Ga]Ga-PSMA-11; metastasis-directed therapy; prostate cancer.
Conflict of interest statement
S.M. received speaker honoraria from General Electric and honoraria as a scientific advisory board member from Eli-Lilly. G.F. received honoraria as a scientific advisory board member from Ipsen, Sanofi, BMS, Pfizer, Novartis, MSD, Janssen. E.Z. received honoraria as a scientific advisory board member from Ipsen, MSD, Janssen. The other authors declare they have no conflict of interest.
Figures

References
-
- Cornford P., van den Bergh R., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

