Differentially Expressed Genes and Signaling Pathways Potentially Involved in Primary Resistance to Chemo-Immunotherapy in Advanced-Stage Gastric Cancer Patients
- PMID: 36613445
- PMCID: PMC9820415
- DOI: 10.3390/ijms24010001
Differentially Expressed Genes and Signaling Pathways Potentially Involved in Primary Resistance to Chemo-Immunotherapy in Advanced-Stage Gastric Cancer Patients
Abstract
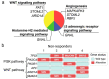
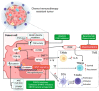
Recently, the combination of chemotherapy plus nivolumab (chemo-immunotherapy) has become the standard of care for advanced-stage gastric cancer (GC) patients. However, despite its efficacy, up to 40% of patients do not respond to these treatments. Our study sought to identify variations in gene expression associated with primary resistance to chemo-immunotherapy. Diagnostic endoscopic biopsies were retrospectively obtained from advanced GC patients previously categorized as responders (R) or non-responders (NR). Thirty-four tumor biopsies (R: n = 16, NR: n = 18) were analyzed by 3′ massive analysis of cDNA ends (3′MACE). We found >30 differentially expressed genes between R and NRs. Subsequent pathway enrichment analyses demonstrated that angiogenesis and the Wnt-β-catenin signaling pathway were enriched in NRs. Concomitantly, we performed next generation sequencing (NGS) analyses in a subset of four NR patients that confirmed alterations in genes that belonged to the Wnt/β-catenin and the phosphoinositide 3-kinase (PI3K) pathways. We speculate that angiogenesis, the Wnt, and the PI3K pathways might offer actionable targets. We also discuss therapeutic alternatives for chemo-immunotherapy-resistant advanced-stage GC patients.
Keywords: PI3K pathway; Wnt pathway; angiogenesis; cancer therapy; gastric cancer; immunotherapy resistance; molecular oncology; β-catenin.
Conflict of interest statement
Garrido (M.G.) has been a member of Scientific Advisory Boards during the past 36 months at Bayer, Novartis, MSD, BMS, Pfizer, Macrogenic, and Merck. MG has been an invited speaker in activities by Novartis, Pfizer, Bayer, BMS, MSD, GBT Biotoscana, and Lilly and has received traveling accommodation and attending support for international meetings by Novartis, MSD, and Bayer. G.I.O. has been an invited speaker on activities organized by Roche and has received traveling accommodations and attending support for international meetings by Roche and MSD. All other authors have no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Morgan E., Arnold M., Camargo M.C., Gini A., Kunzmann A.T., Matsuda T., Meheus F., Verhoeven R.H.A., Vignat J., Laversanne M., et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. eClinicalMedicine. 2022;47:101404. doi: 10.1016/j.eclinm.2022.101404. - DOI - PMC - PubMed
-
- Cordova-Delgado M., Pinto M.P., Retamal I.N., Muñoz-Medel M., Bravo M.L., Fernández M.F., Cisternas B., Mondaca S., Sanchez C., Galindo H., et al. High proportion of potential candidates for immunotherapy in a chilean cohort of gastric cancer patients: Results of the FORCE1 study. Cancers. 2019;11:1275. doi: 10.3390/cancers11091275. - DOI - PMC - PubMed
-
- Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

