A Novel Molecular Signature of Cancer-Associated Fibroblasts Predicts Prognosis and Immunotherapy Response in Pancreatic Cancer
- PMID: 36613599
- PMCID: PMC9820557
- DOI: 10.3390/ijms24010156
A Novel Molecular Signature of Cancer-Associated Fibroblasts Predicts Prognosis and Immunotherapy Response in Pancreatic Cancer
Abstract
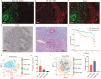
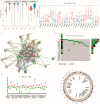
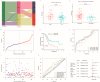
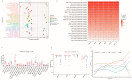
Cancer-associated fibroblasts (CAFs), a prominent population of stromal cells, play a crucial role in tumor progression, prognosis, and treatment response. However, the relationship among CAF-based molecular signatures, clinical outcomes, and tumor microenvironment infiltration remains largely elusive in pancreatic cancer (PC). Here, we collected multicenter PC data and performed integrated analysis to investigate the role of CAF-related genes (CRGs) in PC. Firstly, we demonstrated that α-SMA+ CAFs were the most prominent stromal components and correlated with the poor survival rates of PC patients in our tissue microarrays. Then, we discriminated two diverse molecular subtypes (CAF clusters A and B) and revealed the significant differences in the tumor immune microenvironment (TME), four reported CAF subpopulations, clinical characteristics, and prognosis in PC samples. Furthermore, we analyzed their association with the immunotherapy response of PC patients. Lastly, a CRG score was constructed to predict prognosis, immunotherapy responses, and chemosensitivity in pancreatic cancer patients. In summary, these findings provide insights into further research targeting CAFs and their TME, and they pave a new road for the prognosis evaluation and individualized treatment of PC patients.
Keywords: cancer-associated fibroblasts; molecular signature; pancreatic cancer; therapeutic sensitivity; tumor immune microenvironment.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Figures



References
-
- Helms E., Onate M.K., Sherman M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020;10:648–656. doi: 10.1158/2159-8290.CD-19-1353. - DOI - PMC - PubMed
-
- Neuzillet C., Tijeras-Raballand A., Ragulan C., Cros J., Patil Y., Martinet M., Erkan M., Kleeff J., Wilson J., Apte M., et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 2019;248:51–65. doi: 10.1002/path.5224. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2019YFC1315900/National Key R&D Program of China
- 2018ZHYL0223/Shanghai Municipal Commission of Health and Family Planning
- 20161312/Shanghai Municipal Education Commission-Gao Feng Clinical Medicine
- 21JC1404300/the Scientific and Technological Innovation Project of Science and Technology Commission of Shanghai Municipality
- 81874048/National Natural Science Foundation of China
- 20YF1446400/Shanghai Sailing Program
- Oncology/Shanghai Key Clinical Specialty
- none/Shanghai Leading Talents Project
- none/Innovative research team of high-level local universities in Shanghai
- No. SHDC2020CR1035B/the Clinical Research Plan of SHDC
- 2019CXJQ03/Innovation Group Project of Shanghai Municipal Health Commission
- Y-2019AZZD-0513/Project from CSCO Clinical Oncology Research Foundation
LinkOut - more resources
Full Text Sources
Medical

