Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding
- PMID: 36613608
- PMCID: PMC9820443
- DOI: 10.3390/ijms24010163
Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding
Abstract
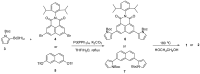
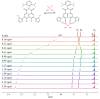
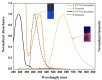
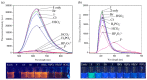
A naphthalene imide (1) and a naphthalene (2) bearing two pyrrole units have been synthesized, respectively, as anion receptors. It was revealed by 1H NMR spectral studies carried out in CD3CN that receptors 1 and 2 bind various anions via hydrogen bonds using both C-H and N-H donors. Compared with receptor 2, receptor 1 shows higher affinity for the test anions because of the enhanced acidity of its pyrrole NH and naphthalene CH hydrogens by the electron-withdrawing imide substituent. Molecular mechanics computations demonstrate that the receptors contact the halide anions via only one of the two respective available N-H and C-H donors whereas they use all four donors for binding of the oxyanions such as dihydrogen phosphate and hydrogen pyrophosphate. Receptor 1, a push-pull conjugated system, displays a strong fluorescence centered at 625 nm, while receptor 2 exhibits an emission with a maximum peak at 408 nm. In contrast, upon exposure of receptors 1 and 2 to the anions in question, their fluorescence was noticeably quenched particularly with relatively basic anions including F-, H2PO4-, HP2O73-, and HCO3-.
Keywords: anion receptor; association constant; fluorescence; hydrogen bond; molecular pincers; titration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Kollman P.A., Allen L.C. The theory of the hydrogen bond. Chem. Rev. 1972;72:283–303. doi: 10.1021/cr60277a004. - DOI
-
- Buckingham A.D., Del Bene J.E., McDowell S.A.C. The hydrogen bond. Chem. Phys. Lett. 2008;463:1–10. doi: 10.1016/j.cplett.2008.06.060. - DOI
-
- Etter M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990;23:120–126. doi: 10.1021/ar00172a005. - DOI
-
- Stahl N., Jencks W.P. Hydrogen bonding between solutes in aqueous solution. J. Am. Chem. Soc. 1986;108:4196–4205. doi: 10.1021/ja00274a058. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

