Hand-Foot-and-Mouth Disease-Associated Enterovirus and the Development of Multivalent HFMD Vaccines
- PMID: 36613612
- PMCID: PMC9820767
- DOI: 10.3390/ijms24010169
Hand-Foot-and-Mouth Disease-Associated Enterovirus and the Development of Multivalent HFMD Vaccines
Abstract
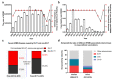
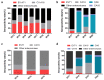
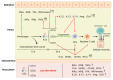
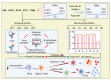
Hand-foot-and-mouth disease (HFMD) is an infectious disease of children caused by more than 20 types of enteroviruses, with most cases recovering spontaneously within approximately one week. Severe HFMD in individual children develops rapidly, leading to death, and is associated with other complications such as viral myocarditis and type I diabetes mellitus. The approval and marketing of three inactivated EV-A71 vaccines in China in 2016 have provided a powerful tool to curb the HFMD epidemic but are limited in cross-protecting against other HFMD-associated enteroviruses. This review focuses on the epidemiological analysis of HFMD-associated enteroviruses since the inactivated EV-A71 vaccine has been marketed, collates the progress in the development of multivalent enteroviruses vaccines in different technical routes reported in recent studies, and discusses issues that need to be investigated for safe and effective HFMD multivalent vaccines.
Keywords: HFMD; multivalent vaccines; systemic immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases.Emerg Microbes Infect. 2016 Jul 20;5(7):e75. doi: 10.1038/emi.2016.73. Emerg Microbes Infect. 2016. PMID: 27436364 Free PMC article. Review.
-
Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease.Expert Rev Vaccines. 2018 Sep;17(9):819-831. doi: 10.1080/14760584.2018.1510326. Epub 2018 Aug 22. Expert Rev Vaccines. 2018. PMID: 30095317 Review.
-
The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China.Emerg Microbes Infect. 2021 Dec;10(1):619-628. doi: 10.1080/22221751.2021.1899772. Emerg Microbes Infect. 2021. PMID: 33682641 Free PMC article.
-
Molecular epidemiology and clinical features of hand, foot and mouth disease requiring hospitalization after the use of enterovirus A71 inactivated vaccine in chengdu, China, 2017-2022: a descriptive study.Emerg Microbes Infect. 2022 Dec;11(1):2510-2519. doi: 10.1080/22221751.2022.2125346. Emerg Microbes Infect. 2022. PMID: 36103331 Free PMC article.
-
The spatial-temporal distribution and etiological characteristics of hand-foot-and-mouth disease before and after EV‑A71 vaccination in Kunming, China, 2017-2020.Sci Rep. 2022 Oct 11;12(1):17028. doi: 10.1038/s41598-022-21312-2. Sci Rep. 2022. PMID: 36220850 Free PMC article.
Cited by
-
Network pharmacology-based exploration identified the antiviral efficacy of Quercetin isolated from mulberry leaves against enterovirus 71 via the NF-κB signaling pathway.Front Pharmacol. 2023 Sep 19;14:1260288. doi: 10.3389/fphar.2023.1260288. eCollection 2023. Front Pharmacol. 2023. PMID: 37795035 Free PMC article.
-
Neutralizing antibody landscape of the non-polio Enteroviruses and future strategy.Front Immunol. 2025 Jan 14;15:1524356. doi: 10.3389/fimmu.2024.1524356. eCollection 2024. Front Immunol. 2025. PMID: 39877351 Free PMC article. Review.
-
A New Human SCARB2 Knock-In Mouse Model for Studying Coxsackievirus A16 and Its Neurotoxicity.Viruses. 2025 Mar 14;17(3):423. doi: 10.3390/v17030423. Viruses. 2025. PMID: 40143350 Free PMC article.
-
Identification of the Emerging C1-like Lineage of Enterovirus A71 in Two Uruguayan Children with Hand-Foot-and-Mouth Disease and Neurological Complications.Viruses. 2024 Nov 8;16(11):1752. doi: 10.3390/v16111752. Viruses. 2024. PMID: 39599865 Free PMC article.
-
T-Cell Receptor Sequences Identify Combined Coxsackievirus-Streptococci Infections as Triggers for Autoimmune Myocarditis and Coxsackievirus-Clostridia Infections for Type 1 Diabetes.Int J Mol Sci. 2024 Feb 1;25(3):1797. doi: 10.3390/ijms25031797. Int J Mol Sci. 2024. PMID: 38339075 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

