A Meta-Analysis Study of SOD1-Mutant Mouse Models of ALS to Analyse the Determinants of Disease Onset and Progression
- PMID: 36613659
- PMCID: PMC9820332
- DOI: 10.3390/ijms24010216
A Meta-Analysis Study of SOD1-Mutant Mouse Models of ALS to Analyse the Determinants of Disease Onset and Progression
Abstract
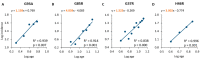
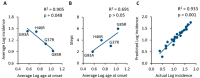
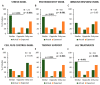
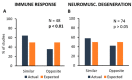
A complex interaction between genetic and external factors determines the development of amyotrophic lateral sclerosis (ALS). Epidemiological studies on large patient cohorts have suggested that ALS is a multi-step disease, as symptom onset occurs only after exposure to a sequence of risk factors. Although the exact nature of these determinants remains to be clarified, it seems clear that: (i) genetic mutations may be responsible for one or more of these steps; (ii) other risk factors are probably linked to environment and/or to lifestyle, and (iii) compensatory plastic changes taking place during the ALS etiopathogenesis probably affect the timing of onset and progression of disease. Current knowledge on ALS mechanisms and therapeutic targets, derives mainly from studies involving superoxide dismutase 1 (SOD1) transgenic mice; therefore, it would be fundamental to verify whether a multi-step disease concept can also be applied to these animal models. With this aim, a meta-analysis study has been performed using a collection of primary studies (n = 137), selected according to the following criteria: (1) the studies should employ SOD1 transgenic mice; (2) the studies should entail the presence of a disease-modifying experimental manipulation; (3) the studies should make use of Kaplan-Meier plots showing the distribution of symptom onset and lifespan. Then, using a subset of this study collection (n = 94), the effects of treatments on key molecular mechanisms, as well as on the onset and progression of disease have been analysed in a large population of mice. The results are consistent with a multi-step etiopathogenesis of disease in ALS mice (including two to six steps, depending on the particular SOD1 mutation), closely resembling that observed in patient cohorts, and revealed an interesting relationship between molecular mechanisms and disease manifestation. Thus, SOD1 mouse models may be considered of high predictive value to understand the determinants of disease onset and progression, as well as to identify targets for therapeutic interventions.
Keywords: ALS epidemiology; ALS pathogenesis; amyotrophic lateral sclerosis; mathematical model; meta-analysis; mouse; superoxide dismutase 1; symptom onset.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sever B., Ciftci H., DeMirci H., Sever H., Ocak F., Yulug B., Tateishi H., Tateishi T., Otsuka M., Fujita M., et al. Comprehensive research on past and future therapeutic strategies devoted to treatment of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2022;23:2400. doi: 10.3390/ijms23052400. - DOI - PMC - PubMed
-
- Loscalzo J., Barabási A.-L., Silverman E.K. Network Medicine: Complex Systems in Human Disease and Therapeutics. Harvard University Press; Cambridge, MA, USA: 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

