Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation-Animal Cell Interaction
- PMID: 36613670
- PMCID: PMC9820676
- DOI: 10.3390/ijms24010226
Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation-Animal Cell Interaction
Abstract
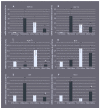
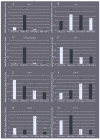
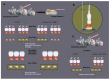
Chemotrophic choice as a metabolic source of energy has characterised animal cell evolution. However, light interactions with animal cell photoacceptors that are able to increase energetic metabolism (photo-biomodulation (PBM)) have been previously described. In the present study, we cut three specimens of Chondrosia reniformis into four equal parts (12 fragments), and we irradiated the regenerating edge of six fragments with the previously characterised 810 nm near-infrared light, delivered at 1 W, 60 J/cm2, 1 W/cm2, and 60 J in a continuous-wave mode for 60 s through a flat-top hand-piece with a rounded spot-size area of 1 cm2. Six fragments were irradiated with 0 W for 60 s as the controls. We performed irradiation at the time 0 h and every 24 h for a total of five administrations. We monitored the regeneration process for five days (120 h) in aquaria by examining the macroscopic and histological changes. We analysed the gene expression profile of the inflammatory processes, apoptosis, heat stress, growth factors, and collagen production and determined oxidative stress enzyme activity and the total prokaryotic symbiont content. PBM sped up C. reniformis regeneration when compared to the controls. Particularly, transforming growth factor TGF3 and TGF6 upregulation during the early phase of regeneration and TGF5 upregulation 120 h postinjury in the irradiated samples supports the positive effect of PBM in sponge tissue recovery. Conversely, the expression of TGF4, a sponge fibroblast growth factor homologue, was not affected by irradiation, indicating that multiple, independent pathways regulate the TGF genes. The results are consistent with our previous data on a wide range of organisms and humans, suggesting that PBM interaction with primary and secondary cell targets has been conserved through the evolution of life forms.
Keywords: Porifera; Wnt; collagen; evolution; heat shock protein; inflammation; light therapy; low-level laser therapy; tissue regeneration; transforming growth factor; tumour necrosis factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Smol’yaninova N.K., Karu T.I., Fedoseeva G.E., Zelenin A.V. Effects of He-Ne Laser Irradiation on Chromatin Properties and Synthesis of Nucleic Acids in Human Peripheral Blood Lymphocytes. Biomed. Sci. 1991;2:121–126. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

