Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats
- PMID: 36613692
- PMCID: PMC9820336
- DOI: 10.3390/ijms24010240
Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats
Abstract
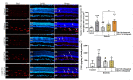
Oxidative stress, neurodegeneration, neuroinflammation, and vascular leakage are believed to play a key role in the early stage of diabetic retinopathy (ESDR). The aim of this study was to investigate the blockade of cannabinoid receptor 1 (CB1R) and activation of cannabinoid receptor 2 (CB2R) as putative therapeutics for the treatment of the early toxic events in DR. Diabetic rats [streptozotocin (STZ)-induced] were treated topically (20 μL, 10 mg/mL), once daily for fourteen days (early stage DR model), with SR141716 (CB1R antagonist), AM1710 (CB2R agonist), and the dual treatment SR141716/AM1710. Immunohistochemical-histological, ELISA, and Evans-Blue analyses were performed to assess the neuroprotective and vasculoprotective properties of the pharmacological treatments on diabetes-induced retinal toxicity. Activation of CB2R or blockade of CB1R, as well as the dual treatment, attenuated the nitrative stress induced by diabetes. Both single treatments protected neural elements (e.g., RGC axons) and reduced vascular leakage. AM1710 alone reversed all toxic insults. These findings provide new knowledge regarding the differential efficacies of the cannabinoids, when administered topically, in the treatment of ESDR. Cannabinoid neuroprotection of the diabetic retina in ESDR may prove therapeutic in delaying the development of the advanced stage of the disease.
Keywords: cannabinoid receptors; early stage diabetic retinopathy; endocannabinoid system; eye drops; neurodegeneration; neuroinflammation; neuroprotection; nitrative stress; vascular leakage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Diabetic Retinopathy—Silently Blinding Millions of People World-Wide. IAPB Vision Atlas. [(accessed on 26 September 2002)]. Available online: http://atlas.iapb.org/vision-trends/diabetic-retinopathy/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

