Chitin Biodegradation by Lytic Polysaccharide Monooxygenases from Streptomyces coelicolor In Vitro and In Vivo
- PMID: 36613716
- PMCID: PMC9820598
- DOI: 10.3390/ijms24010275
Chitin Biodegradation by Lytic Polysaccharide Monooxygenases from Streptomyces coelicolor In Vitro and In Vivo
Abstract
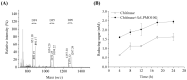
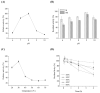
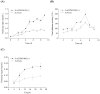
Lytic polysaccharide monooxygenases (LPMOs) have the potential to improve recalcitrant polysaccharide hydrolysis by the oxidizing cleavage of glycosidic bond. Streptomyces species are major chitin decomposers in soil ecological environments and encode multiple lpmo genes. In this study, we demonstrated that transcription of the lpmo gene, Sclpmo10G, in the Streptomyces coelicolor A3(2) (ScA3(2)) strain is strongly induced by chitin. The ScLPMO10G protein was further expressed in Escherichia coli and characterized in vitro. The ScLPMO10G protein showed oxidation activity towards chitin. Chitinase synergy experiments demonstrated that the addition of ScLPMO10G resulted in a substantial in vitro increase in the reducing sugar levels. Moreover, in vivo the LPMO-overexpressing strain ScΔLPMO10G(+) showed stronger chitin-degrading ability than the wild-type, leading to a 2.97-fold increase in reducing sugar level following chitin degradation. The total chitinase activity of ScΔLPMO10G(+) was 1.5-fold higher than that of ScA3(2). In summary, ScLPMO10G may play a role in chitin biodegradation in S. coelicolor, which could have potential applications in biorefineries.
Keywords: Streptomyces coelicolor A3(2); chitin biodegradation; expression; lytic polysaccharide monooxygenases; transcription.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

References
-
- Fernando L.D., Widanage M.C.D., Penfield J., Lipton A.S., Washton N., Latgé J.-P., Wang P., Zhang L., Wang T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls From Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021;8:727053. doi: 10.3389/fmolb.2021.727053. - DOI - PMC - PubMed
-
- Blaak H., Schnellmann J., Walter S., Henrissat B., Schrempf H. Characteristics of an exochitinase from Streptomyces oli-vaceoviridis, its corresponding gene, putative protein domains and relationship to other chitinases. Eur. J. Biochem. 1993;214:659–669. doi: 10.1111/j.1432-1033.1993.tb17966.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

