Childhood Hypophosphatasia Associated with a Novel Biallelic ALPL Variant at the TNSALP Dimer Interface
- PMID: 36613725
- PMCID: PMC9820760
- DOI: 10.3390/ijms24010282
Childhood Hypophosphatasia Associated with a Novel Biallelic ALPL Variant at the TNSALP Dimer Interface
Abstract
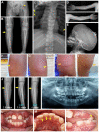
The goal of this study was to perform a clinical and molecular investigation in an eight-year-old female child diagnosed with hypophosphatasia (HPP). The proband and her family were evaluated by medical and dental histories, biochemical analyses, radiographic imaging, and genetic analysis of the tissue-nonspecific alkaline phosphatase (ALPL) gene. A bioinformatic analysis was performed to predict the structural and functional impact of the point mutations in the tissue-nonspecific alkaline phosphatase (TNSALP) molecule and to define their potential contribution to the phenotype. We identified a novel combination of heterozygous ALPL missense variants in the proband, p.Ala33Val and p.Asn47His, compatible with an autosomal recessive mode of inheritance and resulting in skeletal and dental phenotypes. Computational modeling showed that the affected Asn47 residue is located in the coil structure close to the N-terminal α-helix, whereas the affected Ala33 residue is localized in the N-terminal α-helix. Both affected residues are located close to the homodimer interface, suggesting they may impair TNSALP dimer formation and stability. Clinical and biochemical follow-up revealed improvements after six years of ERT. Reporting this novel combination of ALPL variants in childhood HPP provides new insights into genotype-phenotype associations for HPP and specific sites within the TNSALP molecule potentially related to a childhood-onset HPP and skeletal and dental manifestations. Beneficial effects of ERT are implicated in skeletal and dental tissues.
Keywords: 3D modeling; alkaline phosphatase; genotype–phenotype association; hypophosphatasia; premature tooth loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Taillandier A., Domingues C., De Cazanove C., Porquet-Bordes V., Monnot S., Kiffer-Moreira T., Rothenbuhler A., Guggenbuhl P., Cormier C., Baujat G., et al. Molecular diagnosis of hypophosphatasia and differential diagnosis by targeted Next Generation Sequencing. Mol. Genet. Metab. 2015;116:215–220. doi: 10.1016/j.ymgme.2015.09.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

