Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications
- PMID: 36613788
- PMCID: PMC9820311
- DOI: 10.3390/ijms24010345
Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications
Abstract
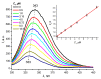
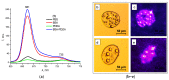
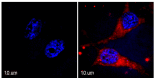
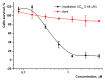
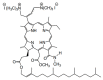
A novel amphiphilic cationic chlorin e6 derivative was investigated as a promising photosensitizer for photodynamic therapy. Two cationic -N(CH3)3+ groups on the periphery of the macrocycle provide additional hydrophilization of the molecule and ensure its electrostatic binding to the mitochondrial membranes and bacterial cell walls. The presence of a hydrophobic phytol residue in the same molecule results in its increased affinity towards the phospholipid membranes while decreasing its stability towards aggregation in aqueous media. In organic media, this chlorin e6 derivative is characterized by a singlet oxygen quantum yield of 55%. Solubilization studies in different polymer- and surfactant-based supramolecular systems revealed the effective stabilization of this compound in a photoactive monomolecular form in micellar nonionic surfactant solutions, including Tween-80 and Cremophor EL. A novel cationic chlorin e6 derivative also demonstrates effective binding towards serum albumin, which enhances its bioavailability and promotes effective accumulation within the target tissues. Laser confocal scanning microscopy demonstrates the rapid intracellular accumulation and distribution of this compound throughout the cells. Together with low dark toxicity and a rather good photostability, this compound demonstrates significant phototoxicity against HeLa cells causing cellular damage most likely through reactive oxygen species generation. These results demonstrate a high potential of this derivative for application in photodynamic therapy.
Keywords: chlorin photosensitizers; intracellular distribution; photodynamic therapy; photostability; phototoxicity; protein binding; singlet oxygen generation; solubilization studies.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Hasan T., Ortel B., Solban N., Pogue B. Photodynamic therapy of cancer. Cancer Med. 2003;7:537–548.
-
- Dos Santos A.F., De Almeida D.R.Q., Terra L.F., Baptista M.S., Labriola L. Photodynamic therapy in cancer treatment-an update review. J. Cancer Metastasis Treat. 2019;5:25. doi: 10.20517/2394-4722.2018.83. - DOI
-
- Waksman R., McEwan P.E., Moore T.I., Pakala R., Kolodgie F.D., Hellinga D.G., Seabron R.C., Rychnovsky S.J., Vasek J., Scott R.W., et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008;52:1024–1032. doi: 10.1016/j.jacc.2008.06.023. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

