Structure of the Flight Muscle Thick Filament from the Bumble Bee, Bombus ignitus, at 6 Å Resolution
- PMID: 36613818
- PMCID: PMC9820631
- DOI: 10.3390/ijms24010377
Structure of the Flight Muscle Thick Filament from the Bumble Bee, Bombus ignitus, at 6 Å Resolution
Abstract
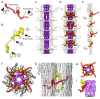
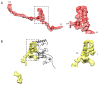
Four insect orders have flight muscles that are both asynchronous and indirect; they are asynchronous in that the wingbeat frequency is decoupled from the frequency of nervous stimulation and indirect in that the muscles attach to the thoracic exoskeleton instead of directly to the wing. Flight muscle thick filaments from two orders, Hemiptera and Diptera, have been imaged at a subnanometer resolution, both of which revealed a myosin tail arrangement referred to as “curved molecular crystalline layers”. Here, we report a thick filament structure from the indirect flight muscles of a third insect order, Hymenoptera, the Asian bumble bee Bombus ignitus. The myosin tails are in general agreement with previous determinations from Lethocerus indicus and Drosophila melanogaster. The Skip 2 region has the same unusual structure as found in Lethocerus indicus thick filaments, an α-helix discontinuity is also seen at Skip 4, but the orientation of the Skip 1 region on the surface of the backbone is less angled with respect to the filament axis than in the other two species. The heads are disordered as in Drosophila, but we observe no non-myosin proteins on the backbone surface that might prohibit the ordering of myosin heads onto the thick filament backbone. There are strong structural similarities among the three species in their non-myosin proteins within the backbone that suggest how one previously unassigned density in Lethocerus might be assigned. Overall, the structure conforms to the previously observed pattern of high similarity in the myosin tail arrangement, but differences in the non-myosin proteins.
Keywords: asynchronous flight muscle; coiled coil; cryoelectron microscopy; myosin; striated muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

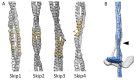


Similar articles
-
Two Forms of Thick Filament in the Flight Muscle of Drosophila melanogaster.Int J Mol Sci. 2024 Oct 21;25(20):11313. doi: 10.3390/ijms252011313. Int J Mol Sci. 2024. PMID: 39457097 Free PMC article.
-
John Squire and the myosin thick filament structure in muscle.J Muscle Res Cell Motil. 2023 Sep;44(3):143-152. doi: 10.1007/s10974-023-09646-4. Epub 2023 Apr 26. J Muscle Res Cell Motil. 2023. PMID: 37099254 Free PMC article. Review.
-
CryoEM structure of Drosophila flight muscle thick filaments at 7 Å resolution.Life Sci Alliance. 2020 Jul 27;3(8):e202000823. doi: 10.26508/lsa.202000823. Print 2020 Aug. Life Sci Alliance. 2020. PMID: 32718994 Free PMC article.
-
Structure of myosin filaments from relaxed Lethocerus flight muscle by cryo-EM at 6 Å resolution.Sci Adv. 2016 Sep 30;2(9):e1600058. doi: 10.1126/sciadv.1600058. eCollection 2016 Sep. Sci Adv. 2016. PMID: 27704041 Free PMC article.
-
Through thick and thin: dual regulation of insect flight muscle and cardiac muscle compared.J Muscle Res Cell Motil. 2019 Jun;40(2):99-110. doi: 10.1007/s10974-019-09536-8. Epub 2019 Jul 10. J Muscle Res Cell Motil. 2019. PMID: 31292801 Free PMC article. Review.
Cited by
-
Predictive analysis of breast cancer response to neoadjuvant chemotherapy through plasma metabolomics.Breast Cancer Res Treat. 2024 Sep;207(2):393-404. doi: 10.1007/s10549-024-07370-2. Epub 2024 May 13. Breast Cancer Res Treat. 2024. PMID: 38740665
-
Two Forms of Thick Filament in the Flight Muscle of Drosophila melanogaster.Int J Mol Sci. 2024 Oct 21;25(20):11313. doi: 10.3390/ijms252011313. Int J Mol Sci. 2024. PMID: 39457097 Free PMC article.
-
Structure of mavacamten-free human cardiac thick filaments within the sarcomere by cryoelectron tomography.Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2311883121. doi: 10.1073/pnas.2311883121. Epub 2024 Feb 22. Proc Natl Acad Sci U S A. 2024. PMID: 38386705 Free PMC article.
-
Structure of the Drosophila melanogaster Flight Muscle Myosin Filament at 4.7 Å Resolution Reveals New Details of Non-Myosin Proteins.Int J Mol Sci. 2023 Oct 5;24(19):14936. doi: 10.3390/ijms241914936. Int J Mol Sci. 2023. PMID: 37834384 Free PMC article.
-
John Squire and the myosin thick filament structure in muscle.J Muscle Res Cell Motil. 2023 Sep;44(3):143-152. doi: 10.1007/s10974-023-09646-4. Epub 2023 Apr 26. J Muscle Res Cell Motil. 2023. PMID: 37099254 Free PMC article. Review.
References
-
- Wendt T., Taylor D., Trybus K.M., Taylor K. Three-Dimensional Image Reconstruction of Dephosphorylated Smooth Muscle Heavy Meromyosin Reveals Asymmetry in the Interaction between Myosin Heads and Placement of Subfragment 2. Proc. Natl. Acad. Sci. USA. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

