HPLC Method Validation for the Estimation of Lignocaine HCl, Ketoprofen and Hydrocortisone: Greenness Analysis Using AGREE Score
- PMID: 36613881
- PMCID: PMC9820389
- DOI: 10.3390/ijms24010440
HPLC Method Validation for the Estimation of Lignocaine HCl, Ketoprofen and Hydrocortisone: Greenness Analysis Using AGREE Score
Abstract
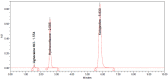
In the current study, the reversed-phased high-pressure liquid chromatography (RP-HPLC) method was proposed for the estimation of lignocaine hydrochloride (LIG), hydrocortisone (HYD) and Ketoprofen (KET) according to International Conference for Harmonization (ICH) Q2 R1 guidelines, in a gel formulation. The chromatographic evaluation was executed using Shimadzu RP-HPLC, equipped with a C8 column and detected using UV at 254 nm wavelength, using acetonitrile and buffer (50:50) as a mobile phase and diluent, at flow rate 1 mL/min and n injection volume of 20 μL. The retention time for LIG, HYD, and KET were 1.54, 2.57, and 5.78 min, correspondingly. The resultant values of analytical recovery demonstrate accuracy and precision of the method and was found specific in identification of the drugs from dosage form and marketed products. The limit of detection (LOD) for LIG, HYD, and KET were calculated to be 0.563, 0.611, and 0.669 ppm, while the limit of quantification (LOQ) was estimated almost at 1.690, 1.833, and 0.223 ppm, respectively. The AGREE software was utilized to evaluate the greenness score of the proposed method, and it was found greener in score (0.76). This study concluded that the proposed method was simple, accurate, precise, robust, economical, reproducible, and suitable for the estimation of drugs in transdermal gels.
Keywords: HPLC Method validation; ICH guidelines; greenness determination; hydrocortisone; ketoprofen; lignocaine HCl; triple drug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nawaz M., Arayne M.S., Sultana N., Haider A., Hisaindee S. Simultaneous determination of fusidic acid and steroids from bulk drugs and human plasma by reversed phase HPLC. Acta Chromatogr. 2014;26:57–66. doi: 10.1556/AChrom.26.2014.1.6. - DOI
-
- Byrne J., Velasco-Torrijos T., Reinhardt R. Development and validation of a novel stability-indicating HPLC method for the simultaneous assay of betamethasone-17-valerate, fusidic acid, potassium sorbate, methylparaben and propylparaben in a topical cream preparation. J. Pharm. Biomed. Anal. 2014;96:111–117. doi: 10.1016/j.jpba.2014.03.005. - DOI - PubMed
-
- Hanif S., Sarfraz R.M., Syed M.A., Mahmood A., Hussain Z. Smart mucoadhesive buccal chitosan/HPMC scaffold for sore throat: In vitro, ex vivo and pharmacokinetic profiling in humans. J. Drug Deliv. Sci. Technol. 2022;71:103271. doi: 10.1016/j.jddst.2022.103271. - DOI
-
- Hanif S., Sarfraz R.M., Syed M.A., Mahmood A., Minhas M.U., Irfan M. Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: A pharmacokinetic investigation among healthy humans. Drug Dev. Ind. Pharm. 2021;47:1209–1222. doi: 10.1080/03639045.2021.1988095. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources