Dendritic Cell-Triggered Immune Activation Goes along with Provision of (Leukemia-Specific) Integrin Beta 7-Expressing Immune Cells and Improved Antileukemic Processes
- PMID: 36613907
- PMCID: PMC9820538
- DOI: 10.3390/ijms24010463
Dendritic Cell-Triggered Immune Activation Goes along with Provision of (Leukemia-Specific) Integrin Beta 7-Expressing Immune Cells and Improved Antileukemic Processes
Abstract
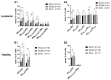
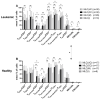
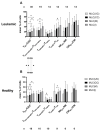
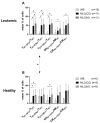
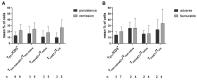
Integrin beta 7 (β7), a subunit of the integrin receptor, is expressed on the surface of immune cells and mediates cell-cell adhesions and interactions, e.g., antitumor or autoimmune reactions. Here, we analyzed, whether the stimulation of immune cells by dendritic cells (of leukemic derivation in AML patients or of monocyte derivation in healthy donors) leads to increased/leukemia-specific β7 expression in immune cells after T-cell-enriched mixed lymphocyte culture-finally leading to improved antileukemic cytotoxicity. Healthy, as well as AML and MDS patients' whole blood (WB) was treated with Kit-M (granulocyte-macrophage colony-stimulating factor (GM-CSF) + prostaglandin E1 (PGE1)) or Kit-I (GM-CSF + Picibanil) in order to generate DCs (DCleu or monocyte-derived DC), which were then used as stimulator cells in MLC. To quantify antigen/leukemia-specific/antileukemic functionality, a degranulation assay (DEG), an intracellular cytokine assay (INTCYT) and a cytotoxicity fluorolysis assay (CTX) were used. (Leukemia-specific) cell subtypes were quantified via flow cytometry. The Kit treatment of WB (compared to the control) resulted in the generation of DC/DCleu, which induced increased activation of innate and adaptive cells after MLC. Kit-pretreated WB (vs. the control) led to significantly increased frequencies of β7-expressing T-cells, degranulating and intracellular cytokine-producing β7-expressing immune cells and, in patients' samples, increased blast lysis. Positive correlations were found between the Kit-M-mediated improvement of blast lysis (vs. the control) and frequencies of β7-expressing T-cells. Our findings indicate that DC-based immune therapies might be able to specifically activate the immune system against blasts going along with increased frequencies of (leukemia-specific) β7-expressing immune cells. Furthermore, β7 might qualify as a predictor for the efficiency and the success of AML and/or MDS therapies.
Keywords: AML; MDS; immune therapy; integrin beta 7; leukemia-derived dendritic cells.
Conflict of interest statement
H.M.S. is involved with Modiblast Pharma GmbH (Oberhaching, Germany), which holds the European Patent 15 801 987.7-1118 and US Patent 15-517627, “Use of immunomodulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias”.
Figures


References
-
- Herold G. Innere Medizin 2020. Walter de Gruyter GmbH & Co KG; Berlin, Germany: 2020.
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Röllig C., Beelen D.W., Braess J., Greil R., Niederwieser D., Passweg J., Reinhardt D., Schlenk R.F. Leitlinie Akute Myeloische Leukämie, Onkopedia, Deutsche Gesellschaft für Hämatologie und Onkologie. [(accessed on 17 November 2020)]. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/akute-myeloische-leuka....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

