Comparative Analysis of Tumor-Associated microRNAs and Tetraspanines from Exosomes of Plasma and Ascitic Fluids of Ovarian Cancer Patients
- PMID: 36613908
- PMCID: PMC9820379
- DOI: 10.3390/ijms24010464
Comparative Analysis of Tumor-Associated microRNAs and Tetraspanines from Exosomes of Plasma and Ascitic Fluids of Ovarian Cancer Patients
Abstract
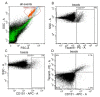
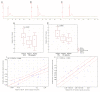
Ovarian cancer (OC) is one of the most common and fatal types of gynecological cancer. In the early phase of OC detection, the current treatment and diagnostic methods are not efficient and sensitive enough. Therefore, it is crucial to explore the mechanisms of OC metastasis and discover valuable factors for early diagnosis of female cancers and novel therapeutic strategies for metastasis. Exosomes are known to be involved in the development, migration, and invasion of cancer cells, and their cargo could be useful for the non-invasive biopsy development. CD151- and Tspan8-positive exosomes are known to support the degradation of the extracellular matrix, and are involved in stroma remodeling, angiogenesis and cell motility, as well as the association of miR-24 and miR-101 with these processes. The objective of this study was to explore the relationship of these components of exosomal cargo, in patients with OC, to clarify the clinical significance of these markers in liquid biopsies. The levels of tetraspanins Tspan8+ and CD151+ exosomes were significantly higher in plasma exosomes of OC patients compared with healthy females (HFs). The relative levels of miR-24 and miR-101 in plasma exosomes of HFs were significantly higher than in plasma exosomes of OC patients, while the levels of these microRNAs in exosomes from plasma and ascites of ill females showed no difference. Our study revealed a strong direct correlation between the change in the ascites exosomes CD151+Tspan8+ subpopulation level and the expression levels of the ascites (R = 0.81, p < 0.05) and plasma exosomal miR-24 (R = 0.74, p < 0.05) in OC patients, which confirms the assumption that exosomal cargo act synergistically to increase cellular motility, affecting cellular processes and signaling. Bioinformatics analysis confirmed the involvement of CD151 and Tspan8 tetraspanins and genes controlled by miR-24-3p and miR-101 in signaling pathways, which are crucial for carcinogenesis, demonstrating that these tetraspanins and microRNAs are potential biomarkers for OC screening, and predictors of poor clinicopathological behavior in tumors.
Keywords: CD151; Tspan8; exosomes; miR-101; miR-24-3p; ovarian cancer; tumor-specific microRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Exosomal Cargo in Ovarian Cancer Dissemination.Curr Issues Mol Biol. 2023 Dec 7;45(12):9851-9867. doi: 10.3390/cimb45120615. Curr Issues Mol Biol. 2023. PMID: 38132461 Free PMC article. Review.
-
The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding.Oncotarget. 2015 Feb 10;6(4):2366-84. doi: 10.18632/oncotarget.2958. Oncotarget. 2015. PMID: 25544774 Free PMC article.
-
Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging.J Ovarian Res. 2022 Feb 19;15(1):27. doi: 10.1186/s13048-022-00961-x. J Ovarian Res. 2022. PMID: 35183243 Free PMC article.
-
Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression.J Exp Clin Cancer Res. 2018 Dec 12;37(1):312. doi: 10.1186/s13046-018-0961-6. J Exp Clin Cancer Res. 2018. Retraction in: J Exp Clin Cancer Res. 2025 Jan 16;44(1):16. doi: 10.1186/s13046-025-03284-z. PMID: 30541597 Free PMC article. Retracted.
-
Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers.Asia Pac J Clin Oncol. 2018 Dec;14(6):383-391. doi: 10.1111/ajco.12869. Epub 2018 Mar 25. Asia Pac J Clin Oncol. 2018. PMID: 29575602 Review.
Cited by
-
Tspan protein family: focusing on the occurrence, progression, and treatment of cancer.Cell Death Discov. 2024 Apr 22;10(1):187. doi: 10.1038/s41420-024-01961-0. Cell Death Discov. 2024. PMID: 38649381 Free PMC article. Review.
-
Special Issue: "Molecular Signatures of Gynecological Cancers-Breast Cancer, Ovarian Cancer, Cervical Cancer, and Endometrial Cancer 2.0".Int J Mol Sci. 2024 Oct 20;25(20):11275. doi: 10.3390/ijms252011275. Int J Mol Sci. 2024. PMID: 39457056 Free PMC article.
-
Research progress of extracellular vesicles in the pathogenesis of type IIIA chronic prostatitis.Front Immunol. 2025 Feb 17;16:1496055. doi: 10.3389/fimmu.2025.1496055. eCollection 2025. Front Immunol. 2025. PMID: 40034709 Free PMC article. Review.
-
Unleashing the potential of urine DNA methylation detection: Advancements in biomarkers, clinical applications, and emerging technologies.Curr Urol. 2025 Sep;19(5):295-302. doi: 10.1097/CU9.0000000000000291. Epub 2025 Jul 19. Curr Urol. 2025. PMID: 40894279 Free PMC article.
-
Exosomal Cargo in Ovarian Cancer Dissemination.Curr Issues Mol Biol. 2023 Dec 7;45(12):9851-9867. doi: 10.3390/cimb45120615. Curr Issues Mol Biol. 2023. PMID: 38132461 Free PMC article. Review.
References
-
- Yunusova N., Kolegova E., Sereda E., Kolomiets L., Villert A., Patysheva M., Rekeda I., Grigor’eva A., Tarabanovskaya N., Kondakova I., et al. Plasma Exosomes of Patients with Breast and Ovarian Tumors Contain an Inactive 20S Proteasome. Molecules. 2021;26:6965. doi: 10.3390/molecules26226965. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

