Sedative Properties of Dexmedetomidine Are Mediated Independently from Native Thalamic Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Function at Clinically Relevant Concentrations
- PMID: 36613961
- PMCID: PMC9820684
- DOI: 10.3390/ijms24010519
Sedative Properties of Dexmedetomidine Are Mediated Independently from Native Thalamic Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Function at Clinically Relevant Concentrations
Abstract
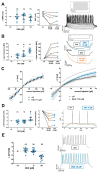
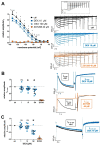
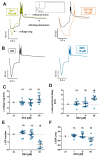
Dexmedetomidine is a selective α2-adrenoceptor agonist and appears to disinhibit endogenous sleep-promoting pathways, as well as to attenuate noradrenergic excitation. Recent evidence suggests that dexmedetomidine might also directly inhibit hyperpolarization-activated cyclic-nucleotide gated (HCN) channels. We analyzed the effects of dexmedetomidine on native HCN channel function in thalamocortical relay neurons of the ventrobasal complex of the thalamus from mice, performing whole-cell patch-clamp recordings. Over a clinically relevant range of concentrations (1-10 µM), the effects of dexmedetomidine were modest. At a concentration of 10 µM, dexmedetomidine significantly reduced maximal Ih amplitude (relative reduction: 0.86 [0.78-0.91], n = 10, and p = 0.021), yet changes to the half-maximal activation potential V1/2 occurred exclusively in the presence of the very high concentration of 100 µM (-4,7 [-7.5--4.0] mV, n = 10, and p = 0.009). Coincidentally, only the very high concentration of 100 µM induced a significant deceleration of the fast component of the HCN activation time course (τfast: +135.1 [+64.7-+151.3] ms, n = 10, and p = 0.002). With the exception of significantly increasing the membrane input resistance (starting at 10 µM), dexmedetomidine did not affect biophysical membrane properties and HCN channel-mediated parameters of neuronal excitability. Hence, the sedative qualities of dexmedetomidine and its effect on the thalamocortical network are not decisively shaped by direct inhibition of HCN channel function.
Keywords: anesthesia; dexmedetomidine; hcn channel; patch-clamp; thalamocortical relay neuron; thalamus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Huupponen E., Maksimow A., Lapinlampi P., Särkelä M., Saastamoinen A., Snapir A., Scheinin H., Scheinin M., Meriläinen P., Himanen S.-L., et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol. Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. - DOI - PubMed
-
- Akeju O., Pavone K.J., Westover M.B., Vazquez R., Prerau M.J., Harrell P.G., Hartnack K.E., Rhee J., Sampson A.L., Habeeb K., et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989. doi: 10.1097/ALN.0000000000000419. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

