Trypanosoma brucei rhodesiense Inhibitor of Cysteine Peptidase (ICP) Is Required for Virulence in Mice and to Attenuate the Inflammatory Response
- PMID: 36614101
- PMCID: PMC9820468
- DOI: 10.3390/ijms24010656
Trypanosoma brucei rhodesiense Inhibitor of Cysteine Peptidase (ICP) Is Required for Virulence in Mice and to Attenuate the Inflammatory Response
Abstract
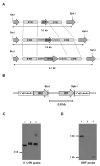
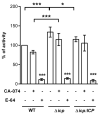
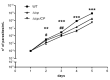
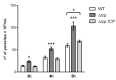
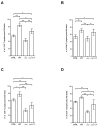
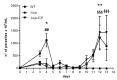
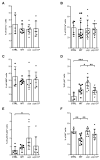
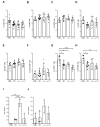
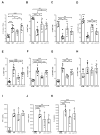
The protozoan Trypanosoma brucei rhodesiense causes Human African Trypanosomiasis, also known as sleeping sickness, and penetrates the central nervous system, leading to meningoencephalitis. The Cathepsin L-like cysteine peptidase of T. b. rhodesiense has been implicated in parasite penetration of the blood-brain barrier and its activity is modulated by the chagasin-family endogenous inhibitor of cysteine peptidases (ICP). To investigate the role of ICP in T. b. rhodesiense bloodstream form, ICP-null (Δicp) mutants were generated, and lines re-expressing ICP (Δicp:ICP). Lysates of Δicp displayed increased E-64-sensitive cysteine peptidase activity and the mutant parasites traversed human brain microvascular endothelial cell (HBMEC) monolayers in vitro more efficiently. Δicp induced E-selectin in HBMECs, leading to the adherence of higher numbers of human neutrophils. In C57BL/6 mice, no Δicp parasites could be detected in the blood after 6 days, while mice infected with wild-type (WT) or Δicp:ICP displayed high parasitemia, peaking at day 12. In mice infected with Δicp, there was increased recruitment of monocytes to the site of inoculation and higher levels of IFN-γ in the spleen. At day 14, mice infected with Δicp exhibited higher preservation of the CD4+, CD8+, and CD19+ populations in the spleen, accompanied by sustained high IFN-γ, while NK1.1+ populations receded nearly to the levels of uninfected controls. We propose that ICP helps to downregulate inflammatory responses that contribute to the control of infection.
Keywords: Trypanosoma; chagasin; inflammation; inhibitor; protease; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Trindade S., Rijo-Ferreira F., Carvalho T., Pinto-Neves D., Guegen F., Aresta-Branco F., Bento F., Young S.A., Pinto A., Abbelee J.V.D., et al. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe. 2016;19:837–848. doi: 10.1016/j.chom.2016.05.002. - DOI - PMC - PubMed
-
- Capewell P., Cren-Travaillé C., Marchesi F., Johnston P., Clucas C., Benson R.A., Gorman T.A., Calvo-Alvarez E., Crouzols A., Jouvion G., et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi: 10.7554/eLife.17716. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

