Polycystin-1 Is a Crucial Regulator of BIN1 Expression and T-Tubule Remodeling Associated with the Development of Dilated Cardiomyopathy
- PMID: 36614108
- PMCID: PMC9820588
- DOI: 10.3390/ijms24010667
Polycystin-1 Is a Crucial Regulator of BIN1 Expression and T-Tubule Remodeling Associated with the Development of Dilated Cardiomyopathy
Abstract
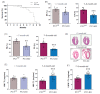
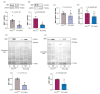
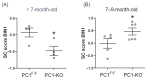
Cardiomyopathy is commonly observed in patients with autosomal dominant polycystic kidney disease (ADPKD), even when they have normal renal function and arterial pressure. The role of cardiomyocyte polycystin-1 (PC1) in cardiovascular pathophysiology remains unknown. PC1 is a potential regulator of BIN1 that maintains T-tubule structure, and alterations in BIN1 expression induce cardiac pathologies. We used a cardiomyocyte-specific PC1-silenced (PC1-KO) mouse model to explore the relevance of cardiomyocyte PC1 in the development of heart failure (HF), considering reduced BIN1 expression induced T-tubule remodeling as a potential mechanism. PC1-KO mice exhibited an impairment of cardiac function, as measured by echocardiography, but no signs of HF until 7-9 months of age. Of the PC1-KO mice, 43% died suddenly at 7 months of age, and 100% died after 9 months with dilated cardiomyopathy. Total BIN1 mRNA, protein levels, and its localization in plasma membrane-enriched fractions decreased in PC1-KO mice. Moreover, the BIN1 + 13 isoform decreased while the BIN1 + 13 + 17 isoform was overexpressed in mice without signs of HF. However, BIN1 + 13 + 17 overexpression was not observed in mice with HF. T-tubule remodeling and BIN1 score measured in plasma samples were associated with decreased PC1-BIN1 expression and HF development. Our results show that decreased PC1 expression in cardiomyocytes induces dilated cardiomyopathy associated with diminished BIN1 expression and T-tubule remodeling. In conclusion, positive modulation of BIN1 expression by PC1 suggests a novel pathway that may be relevant to understanding the pathophysiological mechanisms leading to cardiomyopathy in ADPKD patients.
Keywords: BIN1; T-tubule; dilated cardiomyopathy; heart failure; polycystin-1.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
MeSH terms
Substances
Grants and funding
- 1180613/Agencia Nacional de Investigación y Desarrollo
- 11181000/Agencia Nacional de Investigación y Desarrollo
- 1210644/Agencia Nacional de Investigación y Desarrollo
- 1220325/Agencia Nacional de Investigación y Desarrollo
- 1530011/Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias (FONDAP)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

