Fecal Volatile Organic Compounds and Microbiota Associated with the Progression of Cognitive Impairment in Alzheimer's Disease
- PMID: 36614151
- PMCID: PMC9821163
- DOI: 10.3390/ijms24010707
Fecal Volatile Organic Compounds and Microbiota Associated with the Progression of Cognitive Impairment in Alzheimer's Disease
Abstract
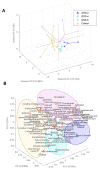
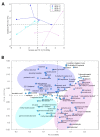
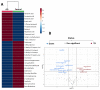
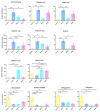
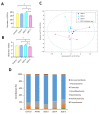
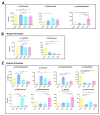
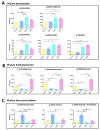
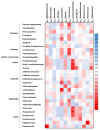
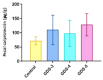
Metabolites produced by an altered gut microbiota might mediate the effects in the brain. Among metabolites, the fecal volatile organic compounds (VOCs) are considered to be potential biomarkers. In this study, we examined both the VOCs and bacterial taxa in the feces from healthy subjects and Alzheimer's disease (AD) patients at early and middle stages. Remarkably, 29 fecal VOCs and 13 bacterial genera were differentiated from the healthy subjects and the AD patients. In general, higher amounts of acids and esters were found in in the feces of the AD patients and terpenes, sulfur compounds and aldehydes in the healthy subjects. At the early stage of AD, the most relevant VOCs with a higher abundance were short-chain fatty acids and their producing bacteria, Faecalibacterium and Lachnoclostridium. Coinciding with the development of dementia in the AD patients, parallel rises of heptanoic acid and Peptococcus were observed. At a more advanced stage of AD, the microbiota and volatiles shifted towards a profile in the feces with increases in hexanoic acid, Ruminococcus and Blautia. The most remarkable VOCs that were associated with the healthy subjects were 4-ethyl-phenol and dodecanol, together with their possible producers Clostridium and Coprococcus. Our results revealed a VOCs and microbiota crosstalk in AD development and their profiles in the feces were specific depending on the stage of AD. Additionally, some of the most significant fecal VOCs identified in our study could be used as potential biomarkers for the initiation and progression of AD.
Keywords: Alzheimer’s disease; cognitive impairment; fecal volatile compounds; gut microbiota; metabolome; short-chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Reisberg B., Ferris S.H., De Leon M.J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry. 1982;139:1136–1139. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

