DNA Methylation-Mediated Overexpression of CXCL1 in Helicobacter pylori-Induced Gastric Cancer: In Silico- and In Vitro-Based Identification of a Potential Biomarker for Carcinogenesis
- PMID: 36614235
- PMCID: PMC9820856
- DOI: 10.3390/ijms24010795
DNA Methylation-Mediated Overexpression of CXCL1 in Helicobacter pylori-Induced Gastric Cancer: In Silico- and In Vitro-Based Identification of a Potential Biomarker for Carcinogenesis
Abstract
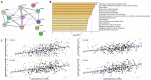
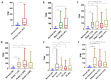
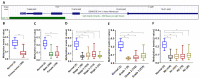
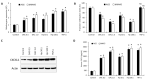
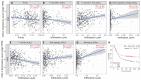
Given the high global prevalence and mortality associated with gastric cancer, and its known causal link with Helicobacter pylori infection, it is important to have a biomarker to identify malignant transformation at early stages. Previously, we, and others, have reported that H. pylori-induced epigenetic changes could mediate carcinogenic transformation of the gastric cells. Also, CXCL1 secreted by gastric cancer cells was reported as a key diagnostic and prognostic biomarker for the pathogenic progression of gastric cancer. In this study, for the first time, we aimed to investigate the role of H. pylori-induced DNA methylation-based epigenetic regulation of CXCL1. In silico analysis of publicly available datasets and in vitro experiments were performed. Our results showed that CXCL1 is highly expressed in both gastric cancer tissues and gastric cancer cells infected with H. pylori. Further, we showed and confirmed that H. pylori-mediated overexpression of CXCL1 is due to hypomethylation of its promoter region. Since epigenetic events such as DNA methylation happen early in the sequence; H. pylori-induced CXCL1 hypomethylation could likely be detected at an early stage of gastric cancer development. Epigenetic modifications, such as CXCL1 hypomethylation, are reversible and could potentially be a therapeutic target using demethylation drugs.
Keywords: CXCL1; DNA methylation; Helicobacter pylori; biomarker; epigenetics; gastric cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Muhammad J.S., Zaidi S.F., Saeed S.A., Ishaq M. Current status of Helicobacter pylori association with haematological and cardiovascular diseases: A mini review. J. Pak. Med. Assoc. 2017;67:907–911. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

