Peptide Drug Conjugates and Their Role in Cancer Therapy
- PMID: 36614268
- PMCID: PMC9820985
- DOI: 10.3390/ijms24010829
Peptide Drug Conjugates and Their Role in Cancer Therapy
Abstract
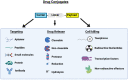
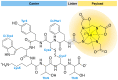
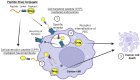
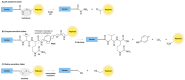
Drug conjugates have become a significant focus of research in the field of targeted medicine for cancer treatments. Peptide-drug conjugates (PDCs), a subset of drug conjugates, are composed of carrier peptides ranging from 5 to 30 amino acid residues, toxic payloads, and linkers that connect the payload to the peptide. PDCs are further broken down into cell-penetrating peptides (CPPs) and cell-targeting peptides (CTPs), each having their own differences in the delivery of cytotoxic payloads. Generally, PDCs as compared to other drug conjugates-like antibody-drug conjugates (ADCs)-have advantages in tumor penetration, ease of synthesis and cost, and reduced off-target effects. Further, as compared to traditional cancer treatments (e.g., chemotherapy and radiation), PDCs have higher specificity for the target cancer with generally less toxic side effects in smaller doses. However, PDCs can have disadvantages such as poor stability and rapid renal clearance due to their smaller size and limited oral bioavailability due to digestion of its peptide structure. Some of these challenges can be overcome with modifications, and despite drawbacks, the intrinsic small size of PDCs with high target specificity still makes them an attractive area of research for cancer treatments.
Keywords: bioconjugates; carrier; linker; payload; peptide-drug conjugates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

