Construction and Validation of a New Naïve Sequestrin Library for Directed Evolution of Binders against Aggregation-Prone Peptides
- PMID: 36614273
- PMCID: PMC9821733
- DOI: 10.3390/ijms24010836
Construction and Validation of a New Naïve Sequestrin Library for Directed Evolution of Binders against Aggregation-Prone Peptides
Abstract
Affibody molecules are small affinity proteins that have excellent properties for many different applications, ranging from biotechnology to diagnostics and therapy. The relatively flat binding surface is typically resulting in high affinity and specificity when developing binding reagents for globular target proteins. For smaller unstructured peptides, the paratope of affibody molecules makes it more challenging to achieve a sufficiently large binding surface for high-affinity interactions. Here, we describe the development of a new type of protein scaffold based on a dimeric form of affibodies with a secondary structure content and mode of binding that is distinct from conventional affibody molecules. The interaction is characterized by encapsulation of the target peptide in a tunnel-like cavity upon binding. The new scaffold was used for construction of a high-complexity phage-displayed library and selections from the library against the amyloid beta peptide resulted in identification of high-affinity binders that effectively inhibited amyloid aggregation.
Keywords: Alzheimer’s disease; Aβ; affibody; directed evolution; phage display; sequestrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

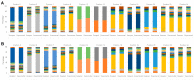


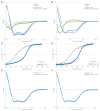

References
-
- Ly H. Lecanemab Confirmatory Phase 3 Clarity AD Study Met Primary Endpoint, Showing Highly Statistically Significant Reduction of Clinical Decline in Large Global Clinical Study of 1,795 Participants with Early Alzheimer’s Disease. [(accessed on 28 September 2022)]. Available online: https://www.eisai.com/news/2022/news202271.html.
-
- Affibody A.B. ACELYRIN, and Inmagene Biopharmaceuticals Announce Data from Global Phase 2 Trial of Izokibep in Patients with Psoriatic Arthritis Presented during 2022 European Alliance of Associations for Rheumatology Congress. [(accessed on 31 October 2022)]. Available online: https://www.affibody.se/affibody-acelyrin-and-inmagene-biopharmaceutical...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

