Choroid Plexus Aquaporins in CSF Homeostasis and the Glymphatic System: Their Relevance for Alzheimer's Disease
- PMID: 36614315
- PMCID: PMC9821203
- DOI: 10.3390/ijms24010878
Choroid Plexus Aquaporins in CSF Homeostasis and the Glymphatic System: Their Relevance for Alzheimer's Disease
Abstract
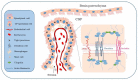
The glymphatic system, a fluid-clearance pathway involved in brain waste clearance, is known to be impaired in neurological disorders, including Alzheimer's disease (AD). For this reason, it is important to understand the specific mechanisms and factors controlling glymphatic function. This pathway enables the flow of cerebrospinal fluid (CSF) into the brain and subsequently the brain interstitium, supported by aquaporins (AQPs). Continuous CSF transport through the brain parenchyma is critical for the effective transport and drainage of waste solutes, such as toxic proteins, through the glymphatic system. However, a balance between CSF production and secretion from the choroid plexus, through AQP regulation, is also needed. Thus, any condition that affects CSF homeostasis will also interfere with effective waste removal through the clearance glymphatic pathway and the subsequent processes of neurodegeneration. In this review, we highlight the role of AQPs in the choroid plexus in the modulation of CSF homeostasis and, consequently, the glymphatic clearance pathway, with a special focus on AD.
Keywords: Alzheimer’s disease; aquaporins; astrocytes; cerebrospinal fluid; choroid plexus; clearance; glymphatic system; homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

