Resistance to Somatostatin Analogs in Italian Acromegaly Patients: The MISS Study
- PMID: 36614826
- PMCID: PMC9821091
- DOI: 10.3390/jcm12010025
Resistance to Somatostatin Analogs in Italian Acromegaly Patients: The MISS Study
Abstract
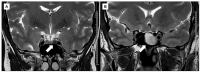
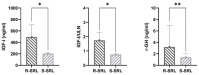
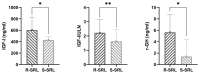
Approximately 60% of acromegaly patients are not adequately controlled by first-generation somatostatin receptor ligands. This multicenter retrospective study aimed to identify the most relevant biomarkers specific for the Italian acromegaly population. Resistant patients were enrolled consecutively based on time of neurosurgery, while responders were collected in a 1:2 ratio. Clinical characteristics and T2-intensity on MRI scans at diagnosis were retrospectively re-evaluated. Histological analyses of CAM5.2 granulation patterns and SSTR2 expression were centrally performed. Sixty-three resistant patients and thirty-three responders were enrolled. A low-grade SSTR2 expression was the most relevant predictor of resistance identified (OR 4.58, p = 0.013), even considering CAM5.2 immunohistochemistry (OR 2.65, p = 0.047). T2-iso/hyperintense pattern on MRI was also associated with a 3.3-fold greater probability of poor response to medical treatment (p = 0.027), as well as a young age at diagnosis (OR 0.96, p = 0.035). In those patients treated only after neurosurgery due to persistent GH-hypersecretion (51, 53.1%) the absence of any appreciable adenomatous remnant on postoperative MRI was associated with a negligible risk of resistance (OR 0.04, p = 0.003). In the Italian acromegaly population, a low-grade SSTR2 expression seems to be the most relevant predictor of resistance to first-generation somatostatin receptor ligands, followed by a SG/intermediate cytokeratin pattern and a T2-iso/hyperintense MRI signal.
Keywords: CAM5.2 granulation pattern; SSTR2; first-generation somatostatin receptor ligands; growth hormone-secreting adenoma; magnetic resonance imaging; precision medicine.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Ezzat S., Caspar-Bell G.M., Chik C.L., Denis M.-C., Domingue M., Imran S.A., Johnson M.D., Lochnan H.A., Nyomba B.L.G., Prebtani A., et al. Predictive Markers for Postsurgical Medical Management of Acromegaly: A Systematic Review and Consensus Treatment Guideline. Endocr. Pract. 2019;25:379–393. doi: 10.4158/EP-2018-0500. - DOI - PubMed
-
- Giustina A., Barkhoudarian G., Beckers A., Ben-Shlomo A., Biermasz N., Biller B., Boguszewski C., Bolanowski M., Bollerslev J., Bonert V., et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020;21:667–678. doi: 10.1007/s11154-020-09588-z. - DOI - PMC - PubMed

