Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort
- PMID: 36614913
- PMCID: PMC9821270
- DOI: 10.3390/jcm12010112
Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort
Abstract
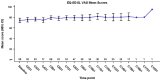
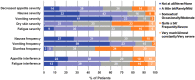
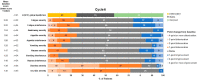
Mobocertinib, an oral, first-in-class epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor selective for EGFR exon 20 insertions (ex20ins), achieved durable responses in adults with previously treated EGFR ex20ins+ metastatic non-small cell lung cancer (mNSCLC) in the EXCLAIM extension cohort of a phase 1/2 study (N = 96; NCT02716116). We assessed patient-reported outcomes (PROs) with mobocertinib 160 mg once daily (28-day cycles) in EXCLAIM (N = 90) with the European Organisation for Research and Treatment of Cancer Core Quality-of-Life Questionnaire (EORTC QLQ-C30) v3.0, lung cancer module (QLQ-LC13), EuroQol-5 Dimensions-5 Levels (EQ-5D-5L) questionnaire, and selected PRO Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) questionnaire. Median treatment duration was 6.8 (range, 0.0-18.8) months (median follow-up: 13.0 [0.7-18.8] months; data cutoff: 1 November 2020). Clinically meaningful improvements in lung cancer symptoms measured by EORTC QLQ-LC13 were observed for dyspnea (54.4% of patients), cough (46.7%), and chest pain (38.9%), evident at cycle 2 and throughout treatment (least-squares mean [LSM] changes from baseline: dyspnea, -3.2 [p = 0.019]; cough, -9.3 [p < 0.001]; chest pain, -8.2 [p < 0.001]). EORTC QLQ-C30 results indicated no statistically significant changes in global health status/quality of life (LSM change from baseline: -1.8 [p = 0.235]). On symptom scores, significant worsening from baseline was observed for diarrhea (LSM change from baseline: +34.1; p < 0.001) and appetite loss (+6.6; p = 0.004), while improvements were observed for dyspnea (LSM change from baseline: -5.1 [p = 0.002]), insomnia (-6.5 [p = 0.001]), and constipation (-5.7 [p < 0.001]). EQ-5D-5L health status was maintained. Common PRO-CTCAE symptoms were diarrhea, dry skin, rash, and decreased appetite (mostly low grade); in the first 24 weeks of treatment, 64.4% of patients had worsening diarrhea frequency and 67.8% had worsening dry skin severity. Overall, PROs with mobocertinib showed clinically meaningful improvement in lung cancer-related symptoms, with health-related quality of life maintained despite changes in some adverse event symptom scales.
Keywords: carcinoma; lung neoplasms; mutation; neoplasm metastasis; non-small cell lung; quality of life.
Conflict of interest statement
Maria Rosario Garcia Campelo: Honoraria—Takeda, AstraZeneca, Roche, Pfizer, Bristol Myers Squibb, and Boehringer Ingelheim; speakers bureau and/or advisory role—Takeda, AstraZeneca, Roche, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Novartis, and Merck, Sharp & Dohme; Caicun Zhou: Honoraria or advisory role—Lilly China, Sanofi, Boehringer Ingelheim, Roche, Merck, Sharp & Dohme, Qilu, Hengrui, Innovent Biologics, C-Stone, LUYE Pharma, TopAlliance Biosciences Inc., and Amoy Diagnostics; Suresh S. Ramalingam: Honoraria or advisory role—Amgen, AstraZeneca, Bristol Myers Squibb, Merck, Lilly, Genentech/Roche, GlaxoSmithKline, and Takeda; research support to institution—Amgen, Advaxis, Bristol Myers Squibb, Genmab, AstraZeneca, and Takeda; Huamao M. Lin: Employment—Takeda; Tae Min Kim: Honoraria or advisory role—AstraZeneca, Boryung, F. Hoffmann-La Roche/Genentech, Janssen, Novartis, Regeneron, Sanofi, Takeda, and Yuhan; research funding outside this work—AstraZeneca–Korea Health Industry Development Institute; Gregory J. Riely: Travel—Merck, Sharp & Dohme; research funding—all to institution: Novartis, Genentech/Roche, Millennium, GlaxoSmithKline, Pfizer, Infinity Pharmaceuticals, ARIAD, Mirati Therapeutics, and Merck; Tarek Mekhail: Speakers bureau—AstraZeneca, Bristol Myers Squibb, Lilly, Merck, and Takeda; advisory role—AstraZeneca and Lilly; Danny Nguyen: Publication fees—Takeda; Erin Goodman: Employment—Takeda; Minal Mehta: Former employment—Takeda; Sanjay Popat: Research funding—all to institution: Boehringer Ingelheim, Epizyme, Bristol Myers Squibb, Clovis Oncology, Roche, Lilly, Daiichi Sankyo, Janssen, Turning Point Therapeutics, and Takeda; honoraria—Boehringer Ingelheim, AstraZeneca, Roche, Takeda, and Chugai Pharma; consulting or advisory role—Boehringer Ingelheim, Bayer, Beigene, Blueprint, Daiichi Sankyo, GlaxoSmithKline, Incyte, Janssen, Lilly, Merck, Takeda, Seattle Genetics, Turning Point Therapeutics, Xcovery, Roche, Novartis, Pfizer, AstraZeneca, Bristol Myers Squibb, Merck, Sharp & Dohme, Guardant Health, and AbbVie; Pasi A. Jänne: Consulting—Araxes Pharmaceuticals, ARIAD/Takeda, AstraZeneca, Boehringer Ingelheim, Chugai, Ignyta, Lilly, Loxo Oncology, Merrimack, Mirati Therapeutics, Pfizer, Roche, Novartis, Voronoi, Daiichi Sankyo, SFJ Pharmaceuticals, and Biocartis; research support—Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Lilly, and Puma Biotechnology; stock ownership—Gatekeeper and Loxo Oncology; postmarketing royalties from Dana-Farber Cancer Institute–owned patent on
Figures

References
-
- Udagawa H., Matsumoto S., Ohe Y., Satouchi M., Furuya N., Kim Y.H., Seto T., Soejima K., Hayakawa D., Kato T., et al. OA07.03 Clinical Outcome of Non-Small Cell Lung Cancer with EGFR/HER2 Exon 20 Insertions Identified in the LC-SCRUM-Japan. J. Thorac. Oncol. 2019;14:S224. doi: 10.1016/j.jtho.2019.08.443. - DOI
-
- Riess J.W., Gandara D.R., Frampton G.M., Madison R., Peled N., Bufill J.A., Dy G.K., Ou S.-H.I., Stephens P.J., McPherson J.D., et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J. Thorac. Oncol. 2018;13:1560–1568. doi: 10.1016/j.jtho.2018.06.019. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

