Advances in the Asymmetric Synthesis of BINOL Derivatives
- PMID: 36615207
- PMCID: PMC9821997
- DOI: 10.3390/molecules28010012
Advances in the Asymmetric Synthesis of BINOL Derivatives
Abstract
BINOL derivatives have shown relevant biological activities and are important chiral ligands and catalysts. Due to these properties, their asymmetric synthesis has attracted the interest of the scientific community. In this work, we present an overview of the most efficient methods to obtain chiral BINOLs, highlighting the use of metal complexes and organocatalysts as well as kinetic resolution. Further derivatizations of BINOLs are also discussed.
Keywords: BINOL; chirality transfer; kinetic resolution; metal-mediated enantioselective coupling; organocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





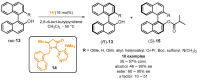
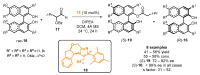
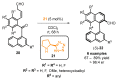
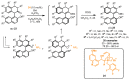


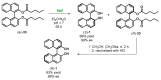


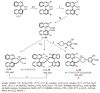
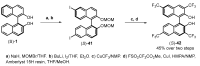

References
-
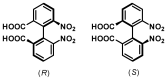
- Christie G.H., Kenner J. LXXI.—The Molecular Configurations of Polynuclear Aromatic Compounds. Part I. The Resolution of γ-6 : 6′-Dinitro- and 4 : 6 : 4′ : 6′-Tetranitro-Diphenic Acids into Optically Active Components. J. Chem. Soc. Trans. 1922;121:614–620. doi: 10.1039/CT9222100614. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

