Effect of Lipophilic Chains on the Antitumor Effect of a Dendritic Nano Drug Delivery System
- PMID: 36615265
- PMCID: PMC9822338
- DOI: 10.3390/molecules28010069
Effect of Lipophilic Chains on the Antitumor Effect of a Dendritic Nano Drug Delivery System
Abstract
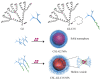
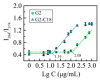
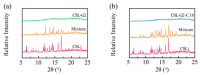
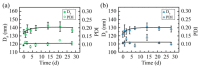
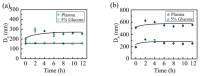
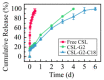
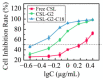
Oligoethylene glycol dendron (G2) has been used in drug delivery due to its unique dendritic structure and excellent properties. In order to investigate the effects of lipophilic chains on drug delivery, the amphiphilic hybrid compound G2-C18 is synthesized, and celastrol (CSL) is selected to prepare "core-shell" structured CSL-G2-C18 nanoparticles (NPs) via the antisolvent precipitation method. Meanwhile, CSL-G2 NPs are prepared as the control. The two NPs show similar particle sizes and polydispersity indexes, while their morphologies exhibit dramatic differences. CSL-G2 NPs are solid spherical particles, while G2-C18 NPs are vesicles. The two NPs present ideal stability and similar release tendencies. The in vitro toxicity results show that the cell inhibition effect of CSL-loaded NPs is significantly enhanced when compared with free CSL, and the antitumor effect of CSL-G2-C18 NPs is stronger than that of CSL-G2 NPs. The IC50 value of CSL-G2 NPs and CSL-G2-C18 NPs is enhanced about 2.8-fold and 5-fold when compared with free CSL, respectively. The above results show that lipophilic chain-linking dendritic hybrid nanocarriers promote antitumor activity by affecting the morphology of NPs, which may aid in the selection of carrier designs.
Keywords: aliphatic chain; amphiphilic nanocarriers; antitumor activity; hybrid structure; morphology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hydroxycamptothecin nanoparticles based on poly/oligo (ethylene glycol): Architecture effects of nanocarriers on antitumor efficacy.Eur J Pharm Biopharm. 2019 Jan;134:178-184. doi: 10.1016/j.ejpb.2018.12.003. Epub 2018 Dec 5. Eur J Pharm Biopharm. 2019. PMID: 30529294
-
A Celastrol Drug Delivery System Based on PEG Derivatives: The Structural Effects of Nanocarriers.Molecules. 2023 Jan 20;28(3):1040. doi: 10.3390/molecules28031040. Molecules. 2023. PMID: 36770710 Free PMC article.
-
Amphiphilic Hybrid Dendritic-Linear Molecules as Nanocarriers for Shape-Dependent Antitumor Drug Delivery.Mol Pharm. 2018 Jul 2;15(7):2665-2673. doi: 10.1021/acs.molpharmaceut.8b00190. Epub 2018 Jun 4. Mol Pharm. 2018. PMID: 29782803
-
Honokiol-Based Nanomedicine Decorated with Ethylene Glycols Derivatives Promotes Antitumor Efficacy.J Biomed Nanotechnol. 2021 Aug 1;17(8):1564-1573. doi: 10.1166/jbn.2021.3126. J Biomed Nanotechnol. 2021. PMID: 34544534
-
Curcumin-Loaded Hybrid Nanoparticles: Microchannel-Based Preparation and Antitumor Activity in a Mouse Model.Int J Nanomedicine. 2021 Jun 17;16:4147-4159. doi: 10.2147/IJN.S303829. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34168445 Free PMC article.
Cited by
-
Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma.Molecules. 2024 Sep 16;29(18):4405. doi: 10.3390/molecules29184405. Molecules. 2024. PMID: 39339402 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

