Fractionation Coupled to Molecular Networking: Towards Identification of Anthelmintic Molecules in Terminalia leiocarpa (DC.) Baill
- PMID: 36615275
- PMCID: PMC9822243
- DOI: 10.3390/molecules28010076
Fractionation Coupled to Molecular Networking: Towards Identification of Anthelmintic Molecules in Terminalia leiocarpa (DC.) Baill
Abstract
Terminalia leiocarpa is a medicinal plant widely used in ethnoveterinary medicine to treat digestive parasitosis whose extracts were shown to be active against gastrointestinal nematodes of domestic ruminants. The objective of our study was to identify compounds responsible for this activity. Column fractionation was performed, and the activity of the fractions was assessed in vitro on Haemonchus contortus and Caenorhabditis elegans as well as their cytotoxicity on WI38 fibroblasts. Two fractions were the most active on both nematode models and less cytotoxic. LC-MS/MS analysis and manual dereplication coupled to molecular networking allowed identification of the main compounds: ellagic acid and derivatives, gallic acid, astragalin, rutin, quinic acid, and fructose. Other potentially identified compounds such as shikimic acid, 2,3-(S)-hexahydroxydiphenoyl-D-glucose or an isomer, quercetin-3-O-(6-O-galloyl)-β-D-galactopyranoside or an isomer, and a trihydroxylated triterpenoid bearing a sugar as rosamultin are reported in this plant for the first time. Evaluation of the anthelmintic activity of the available major compounds showed that ellagic and gallic acids were the most effective in inhibiting the viability of C. elegans. Their quantification in fractions 8 and 9 indicated the presence of about 8.6 and 7.1 µg/mg ellagic acid and about 9.6 and 2.0 µg/mg gallic acid respectively. These concentrations are not sufficient to justify the activity observed. Ellagic acid derivatives and other compounds that were found to be positively correlated with the anthelmintic activity of the fractions may have additive or synergistic effects when combined, but other unidentified compounds could also be implicated in the observed activity.
Keywords: Caenorhabditis elegans; Haemonchus contortus; Terminalia leiocarpa; anthelminthic activity; ellagic acid; gallic acid; molecular networking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

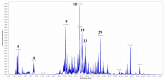
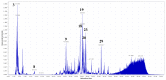


References
-
- Waterman C., Patel Z., Kim S., Rivera A., Pontiggia L., Grace M.H., Smith R. Anthelmintic Activity of Punicalagin from Anogeissus leiocarpus. Univers. J. Plant Sci. 2015;3:67–71. doi: 10.13189/ujps.2015.030402. - DOI
-
- Salih E.Y.A., Julkunen-Tiitto R., Luukkanen O., Sipi M., Fahmi M.K.M., Fyhrquist P.J. Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics. 2020;9:25. doi: 10.3390/antibiotics9070364. - DOI - PMC - PubMed
-
- Tchetan E., Olounlade A.P., Houehanou T.D., Azando E.V.B., Kaneho J.A., Houinato M.R.B., Hounzangbe-Adote S.M., Quetin-Leclercq J., Gbaguidi F.A. Ethnoveterinary Knowledge of Sheep and Goat Farmers in Benin (West Africa): Effect of Socioeconomic and Environmental Factors. Heliyon. 2021;7:e07656. doi: 10.1016/j.heliyon.2021.e07656. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

