Cannabidiol as Self-Assembly Inducer for Anticancer Drug-Based Nanoparticles
- PMID: 36615306
- PMCID: PMC9822096
- DOI: 10.3390/molecules28010112
Cannabidiol as Self-Assembly Inducer for Anticancer Drug-Based Nanoparticles
Abstract
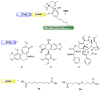
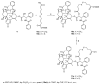
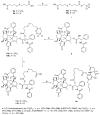
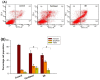
Cannabidiol (CBD) is a biologically active compound present in the plants of the Cannabis family, used as anticonvulsant, anti-inflammatory, anti-anxiety, and more recently, anticancer drug. In this work, its use as a new self-assembly inducer in the formation of nanoparticles is validated. The target conjugates are characterized by the presence of different anticancer drugs (namely N-desacetyl thiocolchicine, podophyllotoxin, and paclitaxel) connected to CBD through a linker able to improve drug release. These nanoparticles are formed via solvent displacement method, resulting in monodisperse and stable structures having hydrodynamic diameters ranging from 160 to 400 nm. Their biological activity is evaluated on three human tumor cell lines (MSTO-211H, HT-29, and HepG2), obtaining GI50 values in the low micromolar range. Further biological assays were carried out on MSTO-211H cells for the most effective NP 8B, confirming the involvement of paclitaxel in cytotoxicity and cell death mechanism.
Keywords: anticancer drug; cannabidiol; self-assembled nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Borrelli S., Christodoulou M.S., Ficarra I., Silvani A., Cappelletti G., Cartelli D., Damia G., Ricci F., Zucchetti M., Dosio F., et al. New Class of Squalene-Based Releasable Nanoassemblies of Paclitaxel, Podophyllotoxin, Camptothecin and Epothilone A. Eur. J. Med. Chem. 2014;85:179–190. doi: 10.1016/j.ejmech.2014.07.035. - DOI - PubMed
-
- Borrelli S., Cartelli D., Secundo F., Fumagalli G., Christodoulou M.S., Borroni A., Perdicchia D., Dosio F., Milla P., Cappelletti G., et al. Self-Assembled Squalene-Based Fluorescent Heteronanoparticles. ChemPlusChem. 2015;80:47–49. doi: 10.1002/cplu.201402239. - DOI
-
- Fumagalli G., Mazza D., Christodoulou M.S., Damia G., Ricci F., Perdicchia D., Stella B., Dosio F., Sotiropoulou P.A., Passarella D. Cyclopamine-Paclitaxel-Containing Nanoparticles: Internalization in Cells Detected by Confocal and Super-Resolution Microscopy. ChemPlusChem. 2015;80:1380–1383. doi: 10.1002/cplu.201500156. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

