Locking the GFP Fluorophore to Enhance Its Emission Intensity
- PMID: 36615428
- PMCID: PMC9822164
- DOI: 10.3390/molecules28010234
Locking the GFP Fluorophore to Enhance Its Emission Intensity
Abstract
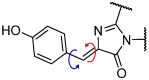
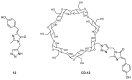
The Green Fluorescent Protein (GFP) and its analogues have been widely used as fluorescent biomarkers in cell biology. Yet, the chromophore responsible for the fluorescence of the GFP is not emissive when isolated in solution, outside the protein environment. The most accepted explanation is that the quenching of the fluorescence results from the rotation of the aryl-alkene bond and from the Z/E isomerization. Over the years, many efforts have been performed to block these torsional rotations, mimicking the environment inside the protein β-barrel, to restore the emission intensity. Molecule rigidification through chemical modifications or complexation, or through crystallization, is one of the strategies used. This review presents an overview of the strategies developed to achieve highly emissive GFP chromophore by hindering the torsional rotations.
Keywords: Z/E isomerization; aggregation-induced emission enhancement; difluoroborate; fluorescence; green fluorescent protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

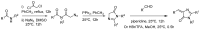

















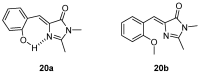
















References
-
- Omary M.A., Patterson H.H. Encyclopedia of Spectroscopy and Spectrometry. Elsevier; Amsterdam, The Netherlands: 2017. Luminescence, Theory; pp. 636–653.
-
- Jabłoński A. Über Den Mechanismus Der Photolumineszenz von Farbstoffphosphoren. Z. Für Phys. 1935;94:38–46. doi: 10.1007/BF01330795. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

