A Novel Curcumin Arginine Salt: A Solution for Poor Solubility and Potential Anticancer Activities
- PMID: 36615455
- PMCID: PMC9822184
- DOI: 10.3390/molecules28010262
A Novel Curcumin Arginine Salt: A Solution for Poor Solubility and Potential Anticancer Activities
Abstract
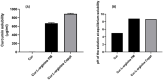
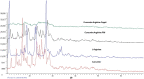
Curcumin is a natural polyphenolic compound with well-known anticancer properties. Poor solubility and permeability hamper its use as an anticancer pharmaceutical product. In this study, L-arginine, a basic amino acid and a small hydrophilic molecule, was utilized to form a salt with the weak acid curcumin to enhance its solubility and potentiate the anticancer activities of curcumin. Two methods were adopted for the preparation of curcumin: L-arginine salt, namely, physical mixing and coprecipitation. The ion pair or salt was characterized for docking, solubility, DSC, FTIR, XRD, in vitro dissolution, and anticancer activities using MCF7 cell lines. The molecular docking suggested a salt/ion-pair complex between curcumin and L-arginine. Curcumin solubility was increased 335- and 440-fold by curcumin in L-arginine, physical, and co-precipitated mixtures, respectively. Thermal and spectral analyses supported the molecular docking and formation of a salt/ion pair between curcumin and L-arginine. The cytotoxicity of curcumin L-arginine salt significantly improved (p < 0.05) by 1.4-fold, as evidenced by the calculated IC50%, which was comparable to Taxol (the standard anticancer drug but with common side effects).
Keywords: L-arginine; breast cancer; curcumin; cytotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

