Dihydrogen Bonding-Seen through the Eyes of Vibrational Spectroscopy
- PMID: 36615456
- PMCID: PMC9822382
- DOI: 10.3390/molecules28010263
Dihydrogen Bonding-Seen through the Eyes of Vibrational Spectroscopy
Abstract
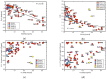
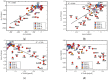
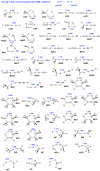
In this work, we analyzed five groups of different dihydrogen bonding interactions and hydrogen clusters with an H3+ kernel utilizing the local vibrational mode theory, developed by our group, complemented with the Quantum Theory of Atoms-in-Molecules analysis to assess the strength and nature of the dihydrogen bonds in these systems. We could show that the intrinsic strength of the dihydrogen bonds investigated is primarily related to the protonic bond as opposed to the hydridic bond; thus, this should be the region of focus when designing dihydrogen bonded complexes with a particular strength. We could also show that the popular discussion of the blue/red shifts of dihydrogen bonding based on the normal mode frequencies is hampered from mode-mode coupling and that a blue/red shift discussion based on local mode frequencies is more meaningful. Based on the bond analysis of the H3+(H2)n systems, we conclude that the bond strength in these crystal-like structures makes them interesting for potential hydrogen storage applications.
Keywords: blue/red shifts; dihydrogen bonding; hydride complexes; hydrogen storage; local vibrational mode analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Grabowski S.J. Understanding Hydrogen Bonds. The Royal Society of Chemistry; London, UK: 2021. (Theoretical and Computational Chemistry Series).
-
- Arunan E. One Hundred Years After The Latimer and Rodebush Paper, Hydrogen Bonding Remains an Elephant! Indian J. Sci. 2020;100:249–255. doi: 10.1007/s41745-019-00154-4. - DOI
LinkOut - more resources
Full Text Sources

