Effects of Histamine and the α-Tocopherol Metabolite α-13'-COOH in an Atopic Dermatitis Full-Thickness Skin Model
- PMID: 36615633
- PMCID: PMC9824170
- DOI: 10.3390/molecules28010440
Effects of Histamine and the α-Tocopherol Metabolite α-13'-COOH in an Atopic Dermatitis Full-Thickness Skin Model
Abstract
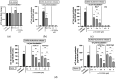
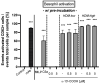
Atopic dermatitis is a T-cell mediated inflammatory skin disease with detected elevated levels of histamine in skin or plasma. In this study, the effects of histamine in a TH2 cytokine environment on human keratinocytes and three-dimensional skin models were investigated. These models were used to explore the anti-inflammatory properties of the α-tocopherol-derived long-chain metabolite α-13'-carboxychromanol (α-13'-COOH). Histamine and TH2 cytokine-induced proliferation of keratinocytes was studied using a scratch assay. The inflammatory marker interleukin-8 was significantly increased in healthy and TH2 cytokine-stimulated keratinocytes and skin models after histamine treatment. The incubation of full-thickness skin models with TH2 cytokines and histamine resulted in morphological changes in the epidermal layer, interpreted as hyperkeratosis. α-13'-COOH significantly decreased interleukin-8 in these disease-associated skin models. Histological staining of filaggrin showed skin-strengthening effects following α-13'-COOH treatment, without changes in mRNA expression. Cytokeratin 10 mRNA expression tended to be increased in response to α-13'-COOH. Anti-allergic properties of α-13'-COOH were studied by pre-incubation of human leukocytes with α-13'-COOH. This resulted in reduced sulfido-leukotriene synthesis. The hyperproliferation effect of histamine in atopic dermatitis skin models may be of further interest to the study of disease-associated morphological changes. Moreover, α-13'-COOH is a promising natural compound for the treatment of inflammatory skin diseases.
Keywords: anti-allergic; anti-inflammatory; atopic dermatitis full-thickness skin model; histamine; hyperkeratosis; α-13’-carboxychromanol.
Conflict of interest statement
M.W. and S.L. have received a research grant from DSM Nutritional Products GmbH. The funder had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the manuscript. The remaining authors declare no conflicts of interest.
Figures







References
-
- Asher M.I., Montefort S., Björkstén B., Lai C.K., Strachan D.P., Weiland S.K., Williams H. Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood: ISAAC Phases One and Three Repeat Multicountry Cross-Sectional Surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. - DOI - PubMed
-
- Silverberg N.B., Silverberg J.I. Inside out or Outside in: Does Atopic Dermatitis Disrupt Barrier Function or Does Disruption of Barrier Function Trigger Atopic Dermatitis? Cutis. 2015;96:359–361. - PubMed
-
- Buys L.M. Treatment Options for Atopic Dermatitis. Am. Fam. Phys. 2007;75:523–528. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

