RETRACTED: Capacitive Behavior of Aqueous Electrical Double Layer Based on Dipole Dimer Water Model
- PMID: 36615925
- PMCID: PMC9824578
- DOI: 10.3390/nano13010016
RETRACTED: Capacitive Behavior of Aqueous Electrical Double Layer Based on Dipole Dimer Water Model
Retraction in
-
RETRACTED: Yang et al. Capacitive Behavior of Aqueous Electrical Double Layer Based on Dipole Dimer Water Model. Nanomaterials 2023, 13, 16.Nanomaterials (Basel). 2024 Dec 24;15(1):1. doi: 10.3390/nano15010001. Nanomaterials (Basel). 2024. PMID: 39717885 Free PMC article.
Abstract
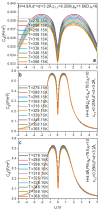
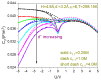
The aim of the present paper is to investigate the possibility of using the dipole dimer as water model in describing the electrical double layer capacitor capacitance behaviors. Several points are confirmed. First, the use of the dipole dimer water model enables several experimental phenomena of aqueous electrical double layer capacitance to be achievable: suppress the differential capacitance values gravely overestimated by the hard sphere water model and continuum medium water model, respectively; reproduce the negative correlation effect between the differential capacitance and temperature, insensitivity of the differential capacitance to bulk electrolyte concentration, and camel-shaped capacitance-voltage curves; and more quantitatively describe the camel peak position of the capacitance-voltage curve and its dependence on the counter-ion size. Second, we fully illustrate that the electric dipole plays an irreplaceable role in reproducing the above experimentally confirmed capacitance behaviors and the previous hard sphere water model without considering the electric dipole is simply not competent. The novelty of the paper is that it shows the potential of the dipole dimer water model in helping reproduce experimentally verified aqueous electric double layer capacitance behaviors. One can expect to realize this potential by properly selecting parameters such as the dimer site size, neutral interaction, residual dielectric constant, etc.
Keywords: aqueous electrolyte; differential capacitance; electric dipole; electrical double layer capacitor; nanoconfinement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lethesh K.C., Bamgbopa M.O., Susantyoko R.A. Prospects and Design Insights of Neat Ionic Liquids as Supercapacitor Electrolytes. Front. Energy Res. 2021;9:741772. doi: 10.3389/fenrg.2021.741772. - DOI
-
- Schütter C., Pohlmann S., Balducci A. Industrial Requirements of Materials for Electrical Double Layer Capacitors: Impact on Current and Future Applications. Adv. Energy Mater. 2019;9:1900334. doi: 10.1002/aenm.201900334. - DOI
-
- Przygocki P., Abbas Q., Gorska B., Beguin F. High-energy hybrid electrochemical capacitor operating down to-40 degrees C with aqueous redox electrolyte based on choline salts. J. Power Sources. 2019;427:283–292. doi: 10.1016/j.jpowsour.2019.04.082. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

