Dissolution Behaviour of Metal-Oxide Nanomaterials in Various Biological Media
- PMID: 36615936
- PMCID: PMC9824292
- DOI: 10.3390/nano13010026
Dissolution Behaviour of Metal-Oxide Nanomaterials in Various Biological Media
Abstract
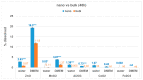
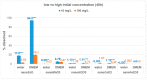
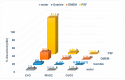
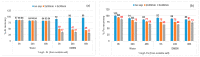
Toxicological effects of metal-oxide-engineered nanomaterials (ENMs) are closely related to their distinct physical-chemical properties, especially solubility and surface reactivity. The present study used five metal-oxide ENMs (ZnO, MnO2, CeO2, Al2O3, and Fe2O3) to investigate how various biologically relevant media influenced dissolution behaviour. In both water and cell culture medium (DMEM), the metal-oxide ENMs were more soluble than their bulk analogues, with the exception that bulk-MnO2 was slightly more soluble in water than nano-MnO2 and Fe2O3 displayed negligible solubility across all tested media (regardless of particle size). Lowering the initial concentration (10 mg/L vs. 100 mg/L) significantly increased the relative solubility (% of total concentration) of nano-ZnO and nano-MnO2 in both water and DMEM. Nano-Al2O3 and nano-CeO2 were impacted differently by the two media (significantly higher % solubility at 10 mg/L in DMEM vs. water). Further evaluation of simulated interstitial lung fluid (Gamble's solution) and phagolysosomal simulant fluid (PSF) showed that the selection of aqueous media significantly affected agglomeration and dissolution behaviour. The solubility of all investigated ENMs was significantly higher in DMEM (pH = 7.4) compared to Gamble's (pH 7.4), attributable to the presence of amino acids and proteins in DMEM. All ENMs showed low solubility in Gamble's (pH = 7.4) compared with PSF (pH = 4.5), attributable to the difference in pH. These observations are relevant to nanotoxicology as increased nanomaterial solubility also affects toxicity. The results demonstrated that, for the purpose of grouping and read-across efforts, the dissolution behaviour of metal-oxide ENMs should be evaluated using aqueous media representative of the exposure pathway being considered.
Keywords: ICP-MS; PSF and Gamble; aluminium oxide; cerium oxide; inhalation pathway; iron oxide; manganese oxide; nanoparticles; zinc oxide.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Piccinno F., Gottschalk F., Seeger S., Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012;14:1109. doi: 10.1007/s11051-012-1109-9. - DOI
-
- Cervantes F.J., Gómez R., Alvarez L.H., Martinez C.M., Hernandez-Montoya V. Efficient anaerobic treatment of synthetic textile wastewater in a UASB reactor with granular sludge enriched with humic acids supported on alumina nanoparticles. Biodegradation. 2015;26:289–298. doi: 10.1007/s10532-015-9734-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

